Limiting Reactant Practice Problems
TLDRThe video script offers a comprehensive guide on identifying limiting reactants and solving stoichiometry problems in chemical reactions. The discussion begins with the reaction between zinc and hydrochloric acid to form hydrogen gas and zinc chloride, illustrating how to determine the limiting reactant by comparing the quantity per coefficient ratio. The video then explores scenarios with varying amounts of reactants and explains how to calculate the theoretical yield of products. Subsequently, the script delves into the reaction between ethane and oxygen to produce carbon dioxide and water, further exemplifying the process of identifying the limiting reactant and calculating the theoretical yield. The summary emphasizes the importance of understanding the limiting reactant as it dictates the maximum quantity of product that can be formed in a reaction, a crucial concept in stoichiometry.
Takeaways
- 🧪 **Limiting Reactant Identification**: The limiting reactant is the substance that runs out first during a chemical reaction and determines the maximum amount of product that can be formed.
- 🔍 **Balancing Chemical Equations**: To identify limiting reactants, chemical equations must be balanced to ensure an accurate stoichiometric relationship between reactants and products.
- ⚖️ **Quantity per Coefficient Ratio**: The reactant with the lowest quantity per coefficient ratio is the limiting reactant, which can be determined by dividing the quantity of the reactant by its stoichiometric coefficient.
- 📉 **Reactant Quantity Comparison**: The limiting reactant is not necessarily the one with the least moles; it depends on the quantity per coefficient ratio after considering the balanced equation.
- 🤔 **Molar Mass Consideration**: When comparing reactants in grams, convert to moles using molar masses to ensure a fair comparison, as different substances have different molar masses.
- 📚 **Stoichiometry Problem-Solving**: In stoichiometry problems, calculate the theoretical yield by determining the amount of product each reactant can produce and comparing these to find the limiting reactant.
- 🔗 **Reactant to Product Conversion**: To find out how much product a reactant can produce, use the molar ratio from the balanced chemical equation to convert moles of reactant to moles of product.
- 📊 **Theoretical Yield Calculation**: The theoretical yield is the maximum amount of product that can be produced in a reaction, which is determined by the limiting reactant.
- ✅ **Two Approaches to Finding Limiting Reactants**: One can find the limiting reactant by either comparing the quantity per coefficient ratio or by calculating the potential product formation from each reactant.
- 🛑 **Reaction Stoppage Point**: The reaction stops when the limiting reactant is completely consumed, even if there is excess of the other reactant.
- 🔬 **Gram to Gram Conversion**: Perform a gram to gram conversion in a three-step process: convert grams to moles using molar mass, use molar ratios to convert between substances, and convert moles of product back to grams.
Q & A
What is the chemical reaction between zinc and aqueous hydrochloric acid?
-Zinc reacts with aqueous hydrochloric acid to produce hydrogen gas and zinc chloride. The balanced chemical equation is Zn + 2HCl → ZnCl2 + H2.
How do you identify the limiting reactant in a chemical reaction?
-The limiting reactant is the one that runs out first in a reaction. It can be identified by comparing the quantity per coefficient ratio of the reactants. The reactant with the lowest ratio is the limiting reactant.
What is the chemical formula for zinc chloride?
-The chemical formula for zinc chloride is ZnCl2, reflecting zinc's +2 charge and the chloride ion's -1 charge.
How do you calculate the moles of a reactant when given the mass and molar mass?
-To calculate the moles of a reactant, divide the mass of the reactant (in grams) by its molar mass.
In part A of the script, why is HCl considered the limiting reactant?
-In part A, HCl is the limiting reactant because it has the lowest quantity per coefficient ratio when comparing the number of atoms of zinc (12) and molecules of HCl (8).
What is the theoretical yield in a chemical reaction?
-The theoretical yield is the maximum amount of product that can be produced in a reaction, based on the limiting reactant.
How do you determine the limiting reactant when given the moles of reactants?
-To determine the limiting reactant when given moles, divide the number of moles of each reactant by its respective coefficient in the balanced chemical equation. The reactant with the lower result is the limiting reactant.
What is the balanced chemical equation for the reaction between ethane and oxygen gas?
-The balanced chemical equation is 2C2H6 + 7O2 → 4CO2 + 6H2O, indicating that two moles of ethane react with seven moles of oxygen to produce four moles of carbon dioxide and six moles of water.
How do you convert grams of one substance to grams of another in a chemical reaction?
-To convert grams to grams, follow these steps: 1) Convert grams of substance A to moles using the molar mass of A. 2) Use the molar ratio to convert from substance A to substance B while keeping the unit the same. 3) Convert moles of substance B to grams using the molar mass of B.
In the reaction between ethane and oxygen, why is oxygen often the limiting reactant?
-Oxygen is often the limiting reactant because it frequently has a higher coefficient in the balanced chemical equation, which means less moles of oxygen are needed to react completely compared to other reactants.
How does the molar mass affect the determination of the limiting reactant?
-The molar mass affects the determination of the limiting reactant because it influences the number of moles present for a given mass of a substance. A substance with a higher molar mass will have fewer moles for the same mass, potentially making it the limiting reactant.
What is the significance of the limiting reactant in predicting the amount of product formed in a reaction?
-The limiting reactant is significant because it determines the maximum amount of product that can be formed in a reaction. Once the limiting reactant is consumed, the reaction stops, and no more product can be produced from the remaining excess reactant(s).
Outlines
🔍 Identifying Limiting Reactants in Stoichiometry
The first paragraph introduces the topic of limiting reactants and stoichiometry problems. It uses the reaction between zinc and aqueous hydrochloric acid to produce hydrogen gas and zinc chloride as an example. The chemical formula for zinc chloride is determined, and the balanced chemical equation is established. The concept of the limiting reactant is explained as the reactant that runs out first. The method to identify it involves calculating the quantity per coefficient ratio, which is done by dividing the number of particles (or moles) by their respective stoichiometric coefficients. Hydrochloric acid (HCl) is identified as the limiting reactant in the given scenarios through this calculation.
🧪 Calculating Theoretical Yields in Reactions
The second paragraph delves into the calculation of theoretical yields, which is the maximum amount of product that can be obtained from a reaction. It uses the reaction between ethane and oxygen to produce carbon dioxide and water as an example. The balanced chemical equation is provided, and the limiting reactant is identified using the quantity per coefficient ratio method. The theoretical yield is calculated by determining how much product each reactant can produce, and the reactant that produces less is the limiting reactant. The example shows that oxygen is the limiting reactant and calculates the theoretical yield of CO2 to be 9.14 moles.
📐 Gram to Gram Conversions and Limiting Reactants
The third paragraph explains the process of gram to gram conversions and how to apply it to determine limiting reactants. It outlines a three-step process involving converting grams to moles, using the molar ratio to convert between substances, and then converting moles back to grams. Using the reaction between ethane and oxygen, the paragraph demonstrates how to find the limiting reactant by comparing the theoretical yields of water produced from both reactants. The calculation shows that oxygen is the limiting reactant, and the theoretical yield of water is 40.5 grams.
🔗 Understanding Theoretical Yield and Limiting Reactants
The fourth paragraph reinforces the concept of theoretical yield and the role of limiting reactants in determining the amount of product formed in a reaction. It emphasizes that the theoretical yield is the maximum quantity of product that can be formed, and it is determined by the limiting reactant. The example provided shows that if all of the oxygen reacts, only 40.5 grams of water will be produced, as oxygen is the limiting reactant. The paragraph also clarifies that the reactant that gives a higher theoretical yield is the excess reactant, as it does not limit the amount of product formed.
Mindmap
Keywords
💡Limiting Reactant
💡Stoichiometry
💡Balanced Chemical Equation
💡Theoretical Yield
💡Molar Mass
💡Mole Ratio
💡Coefficient
💡Quantity per Coefficient Ratio
💡Gram to Gram Conversion
💡Excess Reactant
💡Zinc Chloride
Highlights
The video focuses on limiting reactants and solving stoichiometry problems involving them.
A balanced chemical reaction between zinc and hydrochloric acid is presented, resulting in hydrogen gas and zinc chloride.
Zinc chloride's chemical formula is identified as ZnCl2 based on the charges of zinc and chloride ions.
The concept of the limiting reactant is introduced as the reactant that runs out first in a reaction.
A method to identify the limiting reactant by using the lowest quantity per coefficient ratio is explained.
In part A, HCl is identified as the limiting reactant due to its lower quantity per coefficient ratio compared to zinc.
For part B, the calculation shows that HCl is still the limiting reactant, despite having a greater number of moles.
The ratio of reactants in a reaction is crucial, as two moles of zinc react with four moles of HCl.
In part C, the mass of reactants is converted to moles to determine the limiting reactant, with zinc found to be limiting.
The molar mass differences between zinc and HCl play a significant role in determining the limiting reactant.
A balanced chemical equation for the reaction between ethane and oxygen to produce carbon dioxide and water is provided.
The theoretical yield of a reaction is defined as the maximum amount of product that can be formed.
In part A of the ethane example, oxygen is identified as the limiting reactant, and the theoretical yield of CO2 is calculated.
A practical approach to finding the limiting reactant involves converting the moles of each reactant to the moles of product and comparing.
The limiting reactant can be determined by comparing the theoretical yields of products from each reactant.
In part B of the ethane example, the limiting reactant is found by converting grams to moles and then to grams of product.
The theoretical yield is the lower value obtained from the conversion of reactants to products, indicating the maximum quantity of product.
The importance of understanding the limiting reactant in determining the actual amount of product formed in a reaction is emphasized.
Transcripts
Browse More Related Video
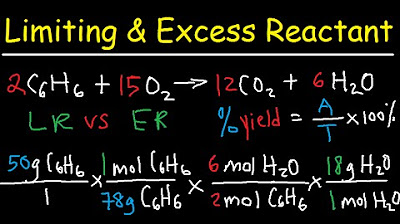
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
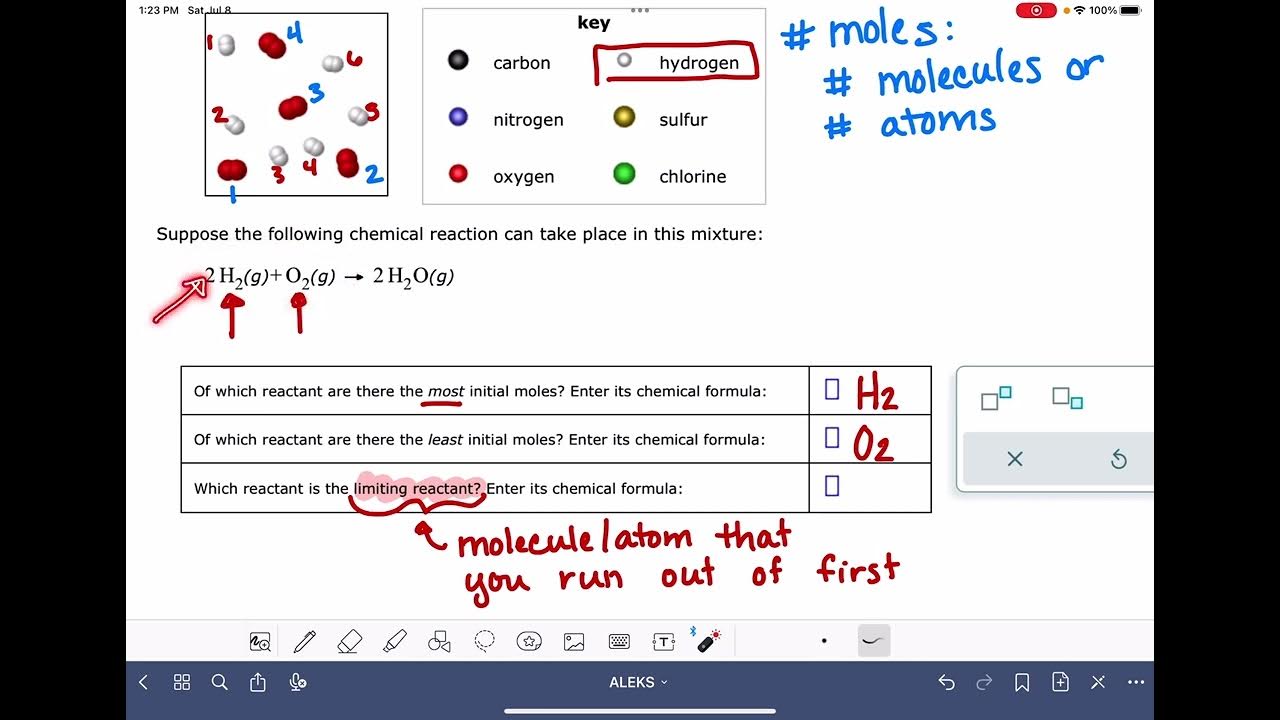
ALEKS: Identifying the limiting reactant in a drawing of a mixture

Introduction to Limiting Reactant and Excess Reactant
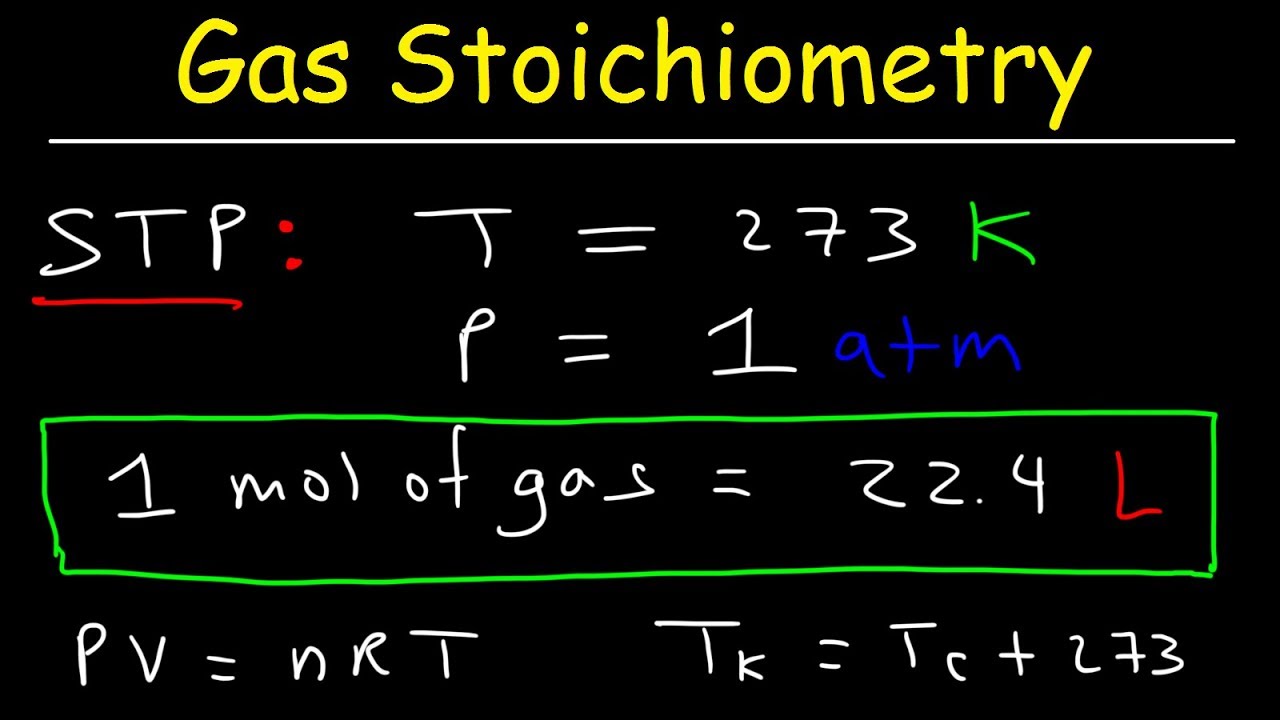
Gas Stoichiometry Problems
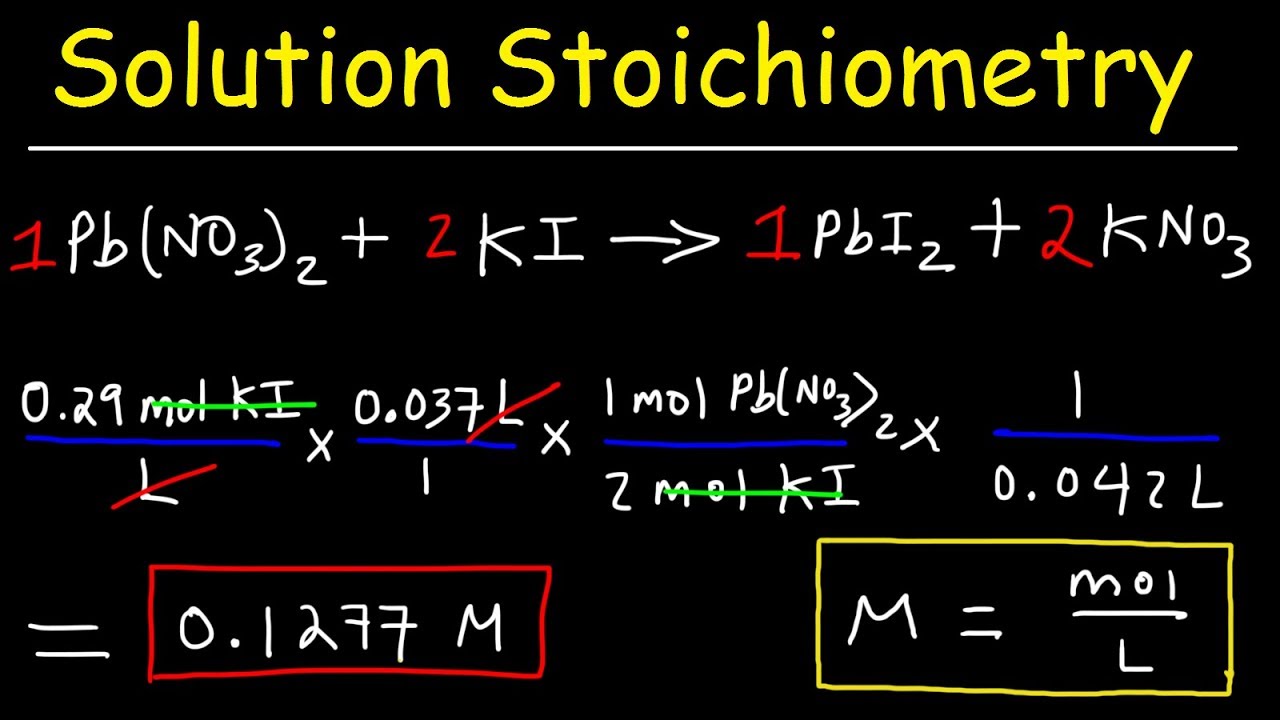
Solution Stoichiometry - Finding Molarity, Mass & Volume
5.0 / 5 (0 votes)
Thanks for rating: