Introduction to Limiting Reactant and Excess Reactant
TLDRThe video script introduces the concept of limiting and excess reactants using an analogy of cooking a cheeseburger, where ingredients represent reactants and the cheeseburger is the product. It explains that the limiting reactant is the first to be used up in a chemical reaction, halting the process and determining the maximum amount of product that can be formed. The script further illustrates this with a bread roll recipe example and a real chemical reaction involving nitrogen and hydrogen to produce ammonia. The importance of understanding limiting reactants for calculating product formation and leftover reactants is emphasized, with a call to practice solving such problems for better comprehension.
Takeaways
- 🍔 The concept of limiting reactant is introduced through an analogy of cooking, comparing chemical reactions to recipes.
- 🥪 Limiting reactant is the substance that will be completely used up first in a chemical reaction, determining the maximum amount of product that can be formed.
- 🥩 In the cheeseburger analogy, meat is the limiting reactant because with only three pieces, only three cheeseburgers can be made before running out.
- 🍞 Excess reactant is the substance left over after the reaction has stopped due to the depletion of the limiting reactant.
- 🥚 The video provides a method for determining the limiting reactant by comparing the ratios of reactants to the amounts available.
- 📈 The process involves calculating what would happen if all of one reactant were used, and then doing the same for another reactant to see which would run out first.
- 🥖 An example with bread rolls demonstrates how to calculate the limiting reactant and excess reactant given certain amounts of flour and water.
- 🧪 The misconception that the limiting reactant is the one present in the smallest amount is clarified as not necessarily true.
- 📊 A chemical equation for producing NH3 (ammonia) is used to illustrate the process of identifying the limiting reactant and calculating the amount of excess reactant left over.
- 🔬 The importance of practice and further study is emphasized for fully understanding the concept of limiting and excess reactants in chemistry.
Q & A
What is a limiting reactant in the context of chemical reactions?
-A limiting reactant is the first reactant that is completely used up in a chemical reaction. Once the limiting reactant is exhausted, no more product can be formed, and the reaction stops.
How does the concept of a limiting reactant relate to cooking a meal?
-The concept of a limiting reactant can be compared to cooking a meal where certain ingredients are required in specific amounts. The ingredient that runs out first limits the number of servings that can be prepared, similar to how a limiting reactant limits the amount of product formed in a chemical reaction.
What is the definition of an excess reactant?
-An excess reactant is the reactant that remains unused after the limiting reactant has been completely consumed in a reaction. It is left over once the reaction stops because the limiting reactant got all used up.
How can you determine the limiting reactant in a given chemical reaction?
-To determine the limiting reactant, you compare the amounts of reactants available and see which one would be completely used up first if the reaction were to continue. The reactant that runs out first is the limiting reactant.
What is the relationship between the coefficients in a chemical equation and the reactants?
-The coefficients in a chemical equation indicate the relative amounts of reactants needed for the reaction. They show the mole ratio of each reactant required to produce the products according to the balanced equation.
How do you calculate the maximum amount of product that can be formed in a reaction?
-To calculate the maximum amount of product, you use the limiting reactant's amount and the stoichiometry from the balanced chemical equation. You determine how much product is formed per mole of the limiting reactant and then multiply by the number of moles of the limiting reactant.
What is a common misconception about limiting reactants?
-A common misconception is that the limiting reactant is the one present in the smallest amount. However, this is not true; the limiting reactant is the one that gets used up first, regardless of its initial quantity.
How do you calculate the amount of excess reactant left over after a reaction?
-To calculate the excess reactant left over, you first determine the limiting reactant. Then, using the stoichiometry of the balanced chemical equation, you calculate how much of the excess reactant would be needed to react with the limiting reactant. Finally, you subtract this amount from the initial quantity of the excess reactant to find out how much is left over.
What is the purpose of using conversion factors in stoichiometry calculations?
-Conversion factors are used in stoichiometry to relate the amounts of reactants and products in a chemical reaction. They help in determining how many moles of one substance are needed for a certain number of moles of another substance, based on the coefficients in the balanced chemical equation.
What is the chemical equation used as an example in the script to illustrate limiting and excess reactants?
-The chemical equation used as an example is N2 + 3H2 → 2NH3. It is used to demonstrate how to calculate the greatest amount of ammonia (NH3) that can be produced with given amounts of nitrogen (N2) and hydrogen (H2), and to identify which is the limiting reactant and the excess reactant.
Why is it important to understand limiting reactants in chemistry?
-Understanding limiting reactants is crucial in chemistry as it helps in predicting the outcome of reactions, optimizing the use of resources, and determining the efficiency of reactions in industrial and laboratory settings. It also aids in the preparation of experiments and the design of chemical processes.
Outlines
🍔 Introduction to Limiting and Excess Reactants with a Cooking Analogy
This paragraph introduces the concept of limiting and excess reactants using a cooking analogy. It compares a chemical reaction to preparing cheeseburgers, where the ingredients (bun, cheese, and meat) are analogous to reactants in chemistry. The limiting reactant is identified as the first ingredient to run out, which in the analogy is the meat, limiting the number of cheeseburgers that can be made. The excess reactant is what remains after the reaction stops, such as the leftover buns and cheese. The paragraph emphasizes the importance of understanding these concepts to solve more complex problems in chemistry.
📝 Solving Limiting Reactant Problems with a Bread Roll Recipe
The second paragraph delves into solving limiting reactant problems using a hypothetical bread roll recipe. It explains how to determine the limiting reactant by comparing the amounts of ingredients (flour and water) available. The process involves calculating the maximum amount of product that can be made from each reactant and identifying which one will be exhausted first. The example clarifies that the limiting reactant is not necessarily the one present in the smallest quantity but the one that runs out first, leading to the conclusion that flour is the limiting reactant in the given scenario.
🧪 Applying the Concept to a Chemical Equation: NH3 Synthesis
This paragraph applies the concept of limiting reactants to a real chemical equation, specifically the synthesis of ammonia (NH3). It demonstrates how to calculate the limiting reactant and the amount of product that can be formed by using the stoichiometry of the chemical equation. The example shows that hydrogen (H2) is the limiting reactant between nitrogen (N2) and hydrogen, and it provides the method to calculate the maximum amount of NH3 that can be produced from the given moles of reactants. The paragraph also introduces the concept of excess reactant, explaining how to determine the amount of the excess reactant left over after the reaction.
📚 Conclusion and Encouragement for Further Study
The final paragraph concludes the discussion on limiting and excess reactants by emphasizing their significance in chemistry. It acknowledges the complexity of the concept and encourages viewers to practice solving problems related to limiting reactants to gain a deeper understanding. The paragraph serves as a call to action for further study and practice to ensure mastery of the topic.
Mindmap
Keywords
💡Limiting Reactant
💡Excess Reactant
💡Chemical Reaction
💡Cooking Analogy
💡Chemical Equation
💡Reactants
💡Products
💡Conversion Factors
💡Stoichiometry
💡Moles
💡Law of Conservation of Mass
Highlights
The video discusses the concept of limiting reactant and excess reactant in the context of chemical reactions, using cooking analogies for better understanding.
A limiting reactant is the first substance to be completely used up in a chemical reaction, which determines the maximum amount of product that can be formed.
Excess reactant refers to the reactant that remains unused after the limiting reactant has been completely consumed, and thus it limits the quantity of the product.
The video uses the analogy of making cheeseburgers to explain the concepts of limiting and excess reactants, where meat is the limiting reactant and buns and cheese are excess reactants.
In the cheeseburger analogy, having more of one ingredient does not mean it is the limiting reactant; it is the one that runs out first.
The video then moves on to a more advanced example using a bread roll recipe to illustrate how to determine the limiting reactant and calculate the excess reactant.
For the bread roll example, flour is the limiting reactant when trying to make the maximum amount of bread rolls with the given ingredients.
The video emphasizes that the limiting reactant is not necessarily the substance you have the least of, but rather the one that gets used up first.
A real chemical equation is used to demonstrate how to calculate the limiting reactant and excess reactant in a chemical reaction, specifically the synthesis of ammonia (NH3).
In the ammonia synthesis example, hydrogen (H2) is identified as the limiting reactant, and nitrogen (N2) is the excess reactant.
The maximum amount of ammonia (NH3) that can be produced from the given moles of N2 and H2 is calculated to be 3.6 moles.
The video provides a step-by-step method for solving limiting reactant problems, including using conversion factors and coefficients from balanced chemical equations.
The concept of limiting reactant is highlighted as one of the most challenging topics in chemistry, and the video encourages viewers to practice and review additional resources for better comprehension.
The video's approach to teaching chemical concepts through everyday analogies aims to make complex topics more accessible and easier to understand.
The importance of identifying the limiting reactant is emphasized as it directly impacts the quantity of the product that can be synthesized in a chemical reaction.
The video concludes with a summary of the process for determining the limiting reactant and calculating the excess reactant, reinforcing the steps outlined throughout the explanation.
Transcripts
Browse More Related Video

How to Find Limiting Reactants | How to Pass Chemistry

Limiting Reactant Practice Problems

Limiting Reagents and Percent Yield
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
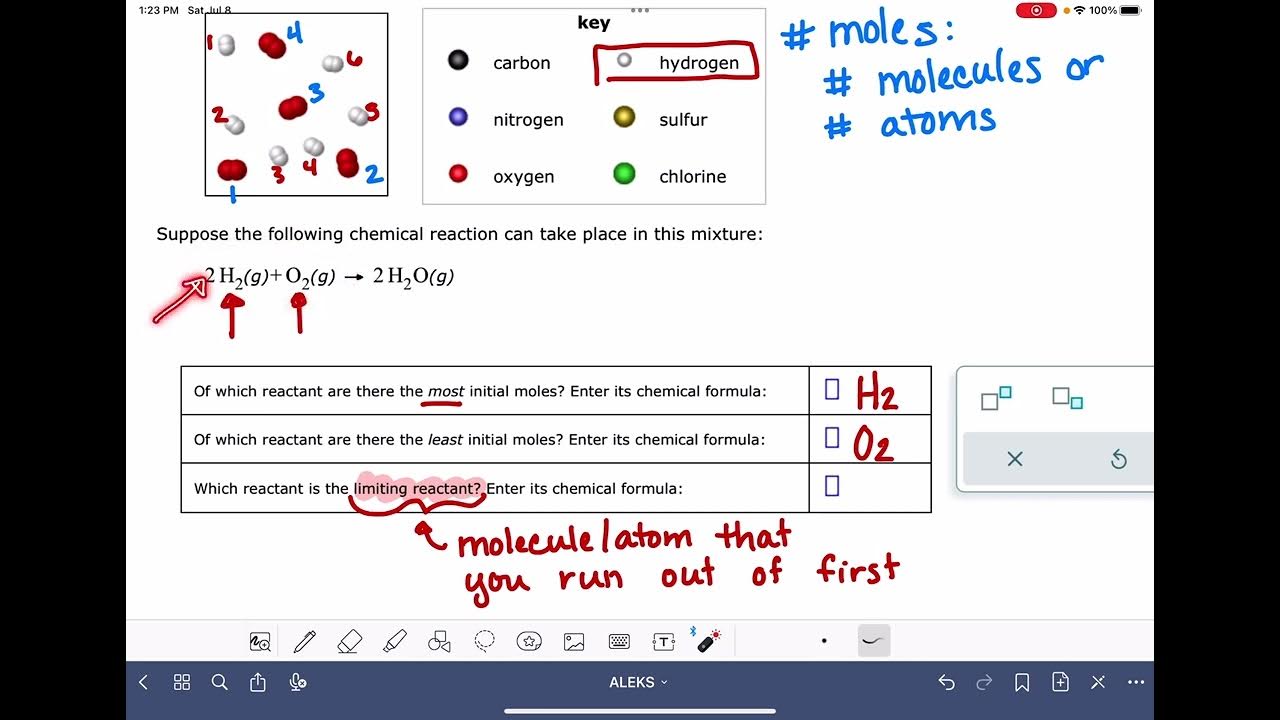
ALEKS: Identifying the limiting reactant in a drawing of a mixture
5.0 / 5 (0 votes)
Thanks for rating: