Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us
TLDRThis educational video script delves into the concept of limiting reactants in chemistry, using a relatable coffee latte analogy to clarify the topic. It guides viewers through the process of identifying the limiting reactant, calculating theoretical yield, and understanding excess reactants. The script emphasizes the importance of stoichiometry, balancing chemical equations, and converting between grams and moles. It also touches on calculating percent yield, a measure of reaction efficiency, and encourages practice for a solid grasp of these fundamental chemistry concepts.
Takeaways
- 🔍 The video discusses the concept of limiting reactants in chemistry, emphasizing that it's a complex topic requiring more than one explanation.
- 📚 The presenter recommends watching multiple videos on the topic to fully grasp the concept of limiting reactants.
- 🛠️ The script uses the analogy of making lattes to explain the idea of limiting reactants, illustrating how the availability of ingredients can limit the production of a product.
- 🧪 It introduces the terms 'limiting reactant' and 'excess reactant', explaining that the limiting reactant is the one that produces the least amount of product in a chemical reaction.
- 📘 The video includes a practice exercise to write and balance a chemical equation for the reaction between iron(II) chloride and sodium phosphate, resulting in iron(II) phosphate and sodium chloride.
- ⚖️ The process of finding the limiting reactant involves converting the mass of reactants to moles, then using stoichiometry to determine which reactant will be depleted first.
- 📊 The script explains how to calculate the theoretical yield of a reaction, which is the amount of product that can be produced by the limiting reactant.
- 🔢 The presenter demonstrates step-by-step calculations to find the limiting reactant and theoretical yield using the given masses of reactants and their respective molar masses.
- 🔄 The script also covers how to determine the amount of excess reactant left after the reaction has reached completion.
- 📈 The concept of percent yield is introduced towards the end of the script, explaining how it is calculated using the actual yield and the theoretical yield.
- 📝 The importance of understanding each step in the process is highlighted, with the presenter advising against memorization and instead promoting a deep understanding of the underlying principles.
Q & A
What is a limiting reactant and why is it important in stoichiometry?
-A limiting reactant, also known as a limiting reagent, is the reactant that is completely consumed in a chemical reaction and determines the maximum amount of product that can be formed. It is important in stoichiometry because understanding the limiting reactant allows you to calculate the theoretical yield of a reaction.
Can you explain the analogy of making lattes to understand limiting reactants?
-The latte analogy compares the concept of limiting reactants to making lattes with brewed coffee and milk. If you have a limited amount of one ingredient (e.g., milk), it will limit the number of lattes you can make, even if you have excess of the other ingredient (brewed coffee). This helps to visualize how the reactant in shortest supply dictates the amount of product formed.
What is the significance of balancing chemical equations in the context of finding the limiting reactant?
-Balancing chemical equations is crucial for determining the stoichiometric ratios between reactants and products. These ratios are used to convert between moles of reactants to moles of products, which is essential for identifying the limiting reactant and calculating theoretical yields.
How do you determine the limiting reactant when given the masses of two reactants?
-You determine the limiting reactant by converting the given masses of each reactant to moles using their respective molar masses. Then, using the stoichiometric ratios from the balanced equation, you calculate how many moles of product each reactant could theoretically produce. The reactant that produces the least amount of product is the limiting reactant.
What is the theoretical yield and how is it related to the limiting reactant?
-The theoretical yield is the maximum amount of product that can be produced in a chemical reaction, based on the stoichiometry of the balanced equation and the amount of limiting reactant. It is directly related to the limiting reactant because it represents the yield based on the reactant that is completely consumed first.
How can you calculate the mass of product formed from a given mass of reactant?
-To calculate the mass of product formed, you first convert the mass of the reactant to moles using its molar mass. Then, apply the stoichiometric ratio from the balanced equation to find the moles of product. Finally, convert the moles of product back to mass using the molar mass of the product.
What is the significance of molar mass in stoichiometric calculations?
-Molar mass is essential in stoichiometric calculations as it allows you to convert between mass and moles for both reactants and products. It is used in each step of the process when transitioning from grams to moles and vice versa.
Can you explain the concept of excess reactant and how it is identified?
-An excess reactant is the reactant that remains after the reaction has completed because it was present in a greater amount than needed to consume the limiting reactant. It is identified by comparing the theoretical yield of products from each reactant; the reactant with the higher theoretical yield is the excess reactant.
How do you calculate the amount of excess reactant remaining after a reaction?
-To calculate the amount of excess reactant remaining, you first determine the amount of product formed from the limiting reactant (theoretical yield). Then, using the stoichiometric ratio from the balanced equation, you calculate the moles of excess reactant that would have reacted. Finally, convert this to mass using the molar mass of the excess reactant and subtract it from the initial mass of the excess reactant.
What is percent yield and how is it calculated?
-Percent yield is a measure of the actual yield of a reaction compared to the theoretical yield. It is calculated by dividing the actual yield by the theoretical yield and multiplying by 100. It is expressed as a percentage and indicates the efficiency of the reaction.
Why is it important to understand the difference between theoretical yield and actual yield?
-Understanding the difference between theoretical yield and actual yield is important because it reflects the efficiency and completeness of a chemical reaction. The theoretical yield is the maximum amount of product that can be produced, while the actual yield is what is physically obtained. Comparing these values can provide insights into reaction conditions, side reactions, and losses during the process.
Outlines
🔍 Understanding Limiting Reactants
The paragraph introduces the concept of limiting reactants in chemistry, which is crucial for determining the maximum amount of product that can be formed in a chemical reaction. It uses the analogy of making lattes with coffee and milk to explain the idea of a limiting ingredient. The paragraph emphasizes the importance of understanding stoichiometry and provides a basic introduction to the concept without going into complex chemical equations.
📚 Balancing Chemical Equations and Identifying Limiting Reactants
This section delves into the process of balancing chemical equations, using the reaction between iron(II) chloride and sodium phosphate as an example. It explains the importance of writing correct chemical formulas and charges for compounds, and then guides through the step-by-step process of balancing the equation. The paragraph also introduces the concept of theoretical yield, which is the amount of product that can be formed theoretically from the limiting reactant.
🧪 Calculating Limiting Reactants and Theoretical Yield
The paragraph explains how to calculate the limiting reactant and theoretical yield in a chemical reaction. It outlines the process of converting grams of reactants to moles, using stoichiometric ratios from the balanced chemical equation, and then back to grams of product. The example provided involves calculating the limiting reactant between iron(II) chloride and sodium phosphate and determining how much sodium chloride can be theoretically produced.
🔢 Detailed Stoichiometry Calculations for Reactants and Products
This section provides a detailed walkthrough of the stoichiometry calculations required to find the limiting reactant and theoretical yield. It includes determining molar masses, converting between grams and moles, and using the balanced chemical equation to find the relationship between reactants and products. The calculations are specific to the given masses of iron(II) chloride and sodium phosphate, leading to the conclusion of which reactant is limiting and the amount of sodium chloride that can be produced.
📉 Determining the Excess Reactant and Its Remaining Quantity
After identifying the limiting reactant and calculating the theoretical yield, this paragraph focuses on determining the excess reactant and how much of it remains after the reaction. It explains the process of using the theoretical yield of the product to back-calculate the amount of excess reactant used in the reaction and then subtracting this from the original amount to find the remaining quantity.
📊 Calculating Percent Yield and Understanding Reaction Efficiency
The final paragraph discusses the concept of percent yield, which measures the efficiency of a chemical reaction by comparing the actual yield to the theoretical yield. It provides a formula for calculating percent yield and explains the importance of understanding both the theoretical and actual yields. The paragraph also emphasizes the significance of the limiting reactant in determining the theoretical yield and the overall success of the reaction.
Mindmap
Keywords
💡Limiting Reactant
💡Stoichiometry
💡Theoretical Yield
💡Excess Reactant
💡Balancing Equations
💡Molar Mass
💡Multiple Ratio
💡Actual Yield
💡Percent Yield
💡Significant Figures
Highlights
Limiting reactant concept explained using a coffee latte analogy.
Importance of understanding limiting reactant in stoichiometry emphasized.
Explanation of the term 'theoretical yield' in chemistry.
Demonstration of writing and balancing chemical equations.
The significance of Roman numerals in indicating the charge of metal in a compound.
Process of converting grams of reactants to moles and then to grams of products.
Use of the balanced chemical equation for stoichiometric calculations.
Method to determine the limiting reactant from given masses of reactants.
Calculation of theoretical yield based on the limiting reactant.
Explanation of the difference between theoretical yield and actual yield.
Process to calculate the amount of excess reactant remaining after the reaction.
Introduction to the concept of percent yield in chemical reactions.
Memorization tips for understanding and remembering chemical formulas and processes.
The necessity of practicing stoichiometry for understanding limiting reactants.
Emphasis on the importance of understanding each step in stoichiometry rather than memorizing.
Explanation of how to calculate the left over amount of excess reactant.
The formula for calculating percent yield and its application in chemistry.
Advice on reviewing and practicing to master the concept of limiting reactants.
Transcripts
Browse More Related Video

Most Common Chemistry Final Exam Question: Limiting Reactants Review

The Limiting Reactant Question That's Found on Most Final Exams | Study Chemistry With Us
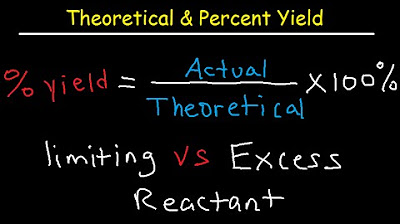
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains
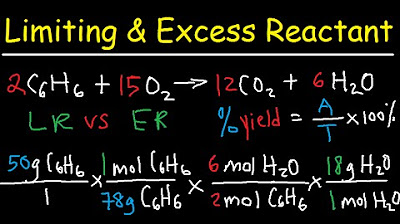
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Practice Problem: Limiting Reagent and Percent Yield

Limiting Reactant Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: