Another Stoichiometry Example in a Solution
TLDRThe video script presents a step-by-step guide to solving a stoichiometry problem involving the reaction of metallic zinc with aqueous hydrochloric acid. The process involves calculating the moles of zinc from its mass, determining the required moles of hydrochloric acid based on the balanced chemical equation, and finally using the given molarity to find the volume of hydrochloric acid solution needed. The solution is detailed, ensuring clarity and understanding, ultimately resulting in the calculation of 144 milliliters of 2.5 molar hydrochloric acid solution required to completely react with 11.8 grams of zinc.
Takeaways
- 🧪 The problem involves a stoichiometry calculation for a reaction between metallic zinc and aqueous hydrochloric acid.
- 📝 The goal is to determine the volume of 0.25 molar hydrochloric acid required to completely convert 11.8 grams of zinc to products.
- 🔍 The first step is to calculate the number of moles of zinc from its given mass using its molar mass (65.38 g/mol).
- 🌐 The balanced chemical equation is verified to ensure the correct stoichiometric ratios.
- 📈 For every mole of zinc, two moles of hydrochloric acid are required based on the balanced equation.
- 🧬 The molarity of the hydrochloric acid solution is given as 2.50 molar, which means 2.50 moles of HCl per liter of solution.
- 📊 The calculation involves setting up a proportion to find the volume of the hydrochloric acid solution needed to provide the required moles of HCl.
- 🔢 The solution volume is initially found in liters and then converted to milliliters by using the conversion factor (1 liter = 1,000 milliliters).
- 🧠 The process emphasizes the importance of understanding and maintaining the correct units throughout the calculation.
- 🎯 The final answer is 144 milliliters of the 2.50 molar hydrochloric acid solution required for the complete reaction with 11.8 grams of zinc.
- 📋 The script provides a detailed walkthrough of the stoichiometry problem, highlighting the step-by-step approach to solving such chemical calculations.
Q & A
What is the reaction taking place in the stoichiometry problem?
-The reaction is between metallic zinc and aqueous hydrochloric acid, occurring in a solution.
What is the solvent in the hydrochloric acid solution?
-The solvent in the hydrochloric acid solution is water.
What is the concentration of the hydrochloric acid solution given in the problem?
-The concentration of the hydrochloric acid solution is 0.25 molar.
How much metallic zinc is used in the problem?
-11.8 grams of metallic zinc is used in the problem.
What is the molar mass of zinc?
-The molar mass of zinc is 65.38 grams per mole.
How many moles of zinc are present in 11.8 grams?
-To find the number of moles of zinc in 11.8 grams, you divide the mass (11.8 grams) by the molar mass (65.38 grams/mole).
What is the stoichiometric ratio of zinc to hydrochloric acid in the reaction?
-The stoichiometric ratio is 1 mole of zinc to 2 moles of hydrochloric acid.
How do you find the moles of hydrochloric acid required for the reaction?
-You multiply the moles of zinc by the stoichiometric ratio (2 moles of HCl per mole of Zn) to find the moles of HCl required.
What is the molarity of the hydrochloric acid solution?
-The molarity of the hydrochloric acid solution is 2.5 moles per liter.
How do you convert the moles of hydrochloric acid to volume?
-You use the molarity (moles per liter) of the solution to find the volume needed by dividing the moles of HCl by the molarity.
What is the final volume of hydrochloric acid solution required to react with 11.8 grams of zinc?
-The final volume required is 144 milliliters of the 2.5 molar hydrochloric acid solution.
How many milliliters are in a liter?
-There are 1,000 milliliters in a liter.
Outlines
🧪 Stoichiometry Problem: Zinc and Hydrochloric Acid Reaction
This paragraph introduces a stoichiometry problem involving the reaction of metallic zinc with aqueous hydrochloric acid. The problem sets up the scenario within a solution, with zinc as the metal reacting agent and hydrochloric acid as the dissolved reactant in water. The objective is to determine the volume of 0.25 molar hydrochloric acid needed to completely convert 11.8 grams of zinc into products. The paragraph outlines the plan to solve the problem by first calculating the moles of zinc from its given mass, then using the stoichiometry of the balanced equation to find the required moles of hydrochloric acid. It emphasizes the need to verify the balance of the chemical equation and explains the process of converting grams to moles using the molar mass of zinc. The paragraph concludes with the intention to calculate the volume of the hydrochloric acid solution based on its molarity.
📈 Solution Calculation and Molarity Application
This paragraph delves into the calculation process for the stoichiometry problem presented in the previous section. It explains the concept of molarity, which is the number of moles of solute per liter of solution, and applies it to find out how many moles are present in a liter of the 2.5 molar hydrochloric acid solution. The paragraph guides through the steps of using the given molarity to calculate the liters of the hydrochloric acid solution needed for the reaction. It then proceeds to convert the volume from liters to milliliters, as the final answer is required in milliliters. The paragraph emphasizes the importance of understanding the unit conversions and the logical steps involved in the calculation, ultimately leading to the solution of requiring 144 milliliters of the 2.5 molar hydrochloric acid solution for the complete reaction of the given mass of zinc.
Mindmap
Keywords
💡stoichiometry
💡metallic zinc
💡aqueous solution
💡molar mass
💡molarity
💡balanced equation
💡moles
💡liters
💡milliliters
💡concentration
💡reaction
Highlights
The stoichiometry problem involves a reaction between metallic zinc and aqueous hydrochloric acid.
The reaction occurs in a solution where hydrochloric acid is dissolved in water, forming an aqueous solution.
The goal is to determine the volume of 0.25 molar hydrochloric acid required to convert 11.8 grams of zinc completely to products.
The problem assumes a balanced chemical equation for the reaction between zinc and hydrochloric acid.
Zinc's atomic weight is 65.38, which is used to calculate its molar mass.
For every mole of zinc, two moles of hydrochloric acid are required based on the balanced equation.
The molarity of the hydrochloric acid solution is given as 2.50 moles per liter.
The problem involves converting grams of zinc to moles and then determining the moles of hydrochloric acid needed.
The volume of the hydrochloric acid solution required is found by relating moles of HCl to its molarity.
The final answer is sought in milliliters, not liters, as specified in the problem.
The calculation involves canceling out unnecessary units to isolate the volume of the solution in milliliters.
The process emphasizes the importance of understanding the conceptual basis behind each step of the calculation.
The solution process is detailed, walking through each step from calculating moles of zinc to the final volume of hydrochloric acid solution.
The final calculated volume of 2.5 molar hydrochloric acid solution required is 144 milliliters.
The problem demonstrates the application of stoichiometry in determining reactants and products in a chemical reaction.
The example showcases the practical use of molarity and molar mass in chemical calculations.
The transcript provides a clear and methodical approach to solving stoichiometry problems, emphasizing the step-by-step process.
Transcripts
Browse More Related Video

Stoichiometry of a Reaction in Solution

Molarity, Solution Stoichiometry and Dilution Problem

GCSE Chemistry - Moles, Concentration & Volume Calculations #29

4.93 | How many milliliters of a 0.1500-M solution of KOH will be required to titrate 40.00 mL of a
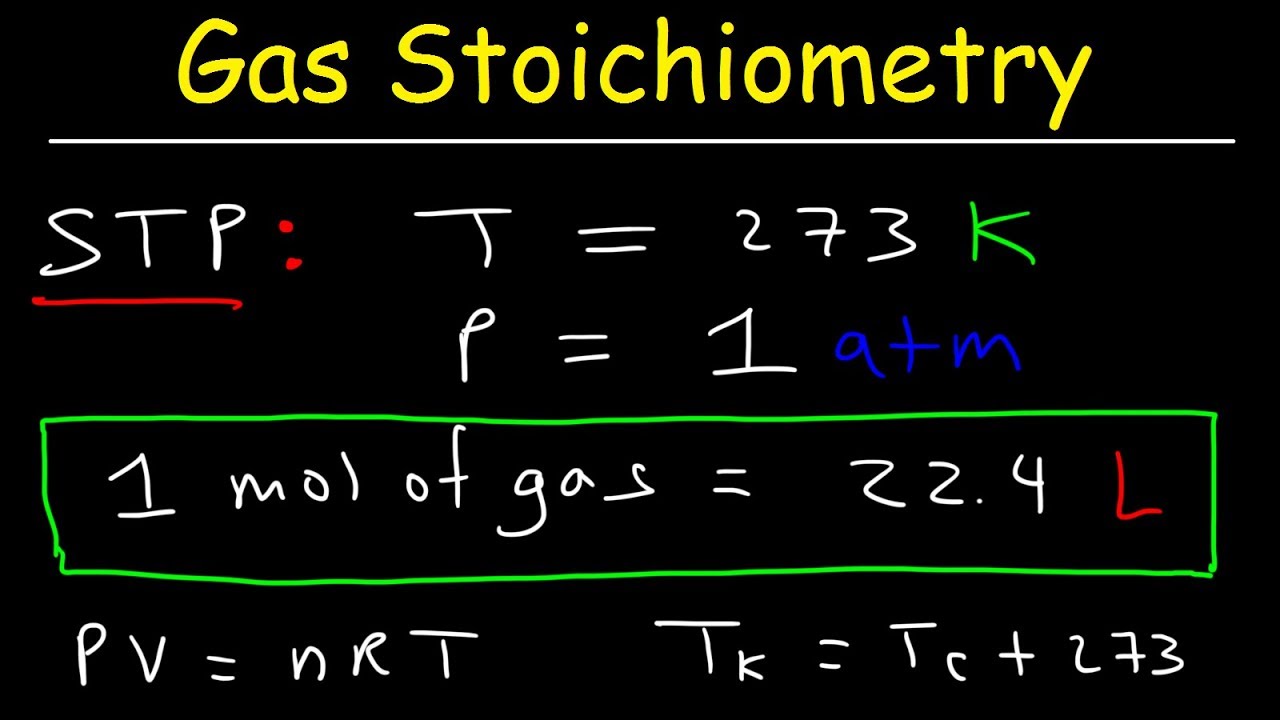
Gas Stoichiometry Problems
BTEC Applied Science: Unit 1 Chemistry Calculating Masses in Reactions
5.0 / 5 (0 votes)
Thanks for rating: