First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
TLDRThe video script discusses the first law of thermodynamics, which states that energy cannot be created or destroyed, only transformed. It uses the example of a ball thrown into the air to illustrate the transformation of kinetic energy to potential energy and back, and further explains how air resistance leads to the conversion of some energy into heat. The concept of internal energy is introduced as a measure of all energy within a system, and the script concludes with an exploration of how changes in internal energy relate to heat transfer and work done by or on a system.
Takeaways
- 📜 The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another.
- 🏐 Examples of energy transformation include throwing a ball up in the air, where its kinetic energy is converted into potential energy at the peak, and then back into kinetic energy as it falls.
- 🔄 When air resistance is present, some of the ball's kinetic energy is transformed into heat due to friction with air molecules, resulting in the ball falling back slower than it was thrown.
- 🌡️ Internal energy is a macroscopic property that represents the total energy within a system, including the kinetic and potential energies of its particles.
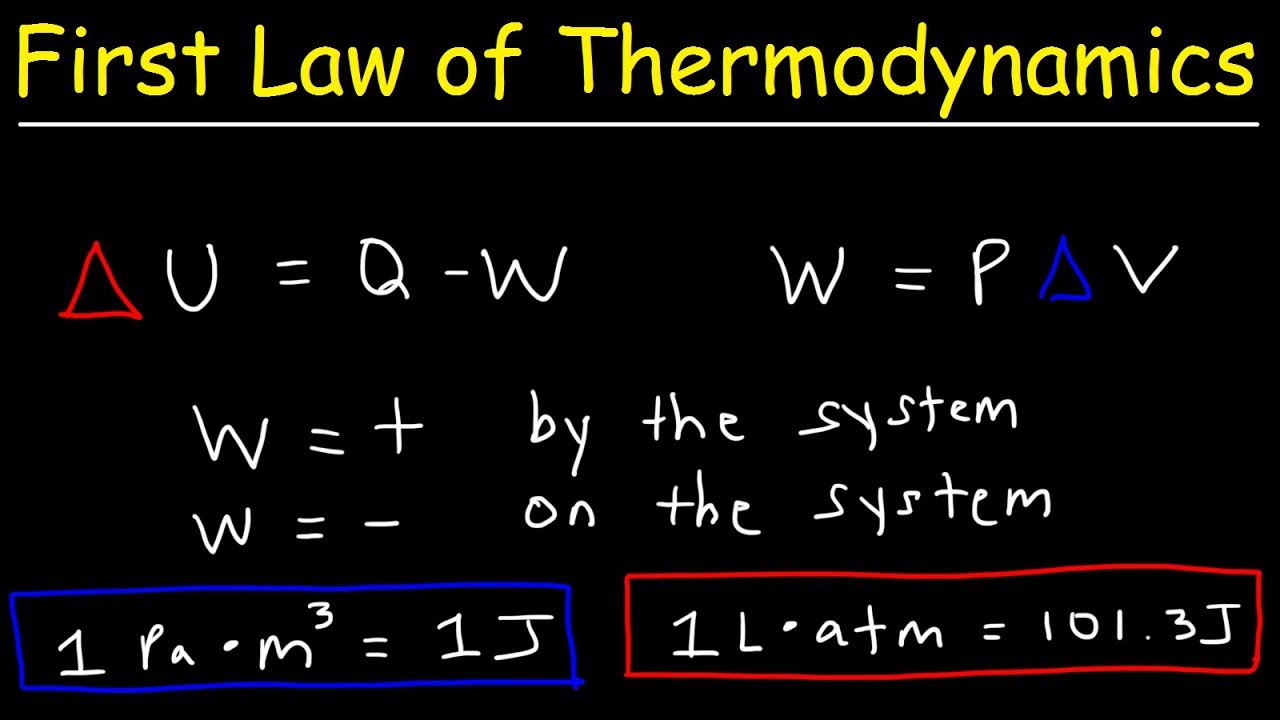
- 🔧 The first law of thermodynamics can be expressed as the change in internal energy (∆U) being equal to the heat added to the system (Q) minus the work done by the system (W).
- ⚙️ In the context of an ideal gas, the internal energy can be simplified to represent mainly the kinetic energy of the particles, as there are no intermolecular bonds to consider.
- 🔄 The transformation of energy can be illustrated through work and heat transfer; work done by the system results in a loss of internal energy, while work done on the system or heat added increases it.
- 📊 The script emphasizes the importance of understanding the various forms of energy and their transformations, as well as the concept of internal energy in thermodynamics.
- 💡 The concept of heat as a form of energy is highlighted, showing how it can be transferred between systems and contribute to the overall energy balance.
- 🔎 The script also introduces various notations that may be encountered in thermodynamics equations, such as ∆U, Q, and W, and encourages a deep understanding of their implications.
Q & A
What is the first law of thermodynamics mentioned in the script?
-The first law of thermodynamics states that energy cannot be created or destroyed, only transformed from one form to another.
How does the script illustrate the first law of thermodynamics with the example of throwing a ball?
-The script uses the example of throwing a ball to demonstrate the transformation of kinetic energy into potential energy and back into kinetic energy, showing that energy is not lost but rather changes forms according to the first law of thermodynamics.
What is the role of air resistance in the energy transformation of the ball?
-Air resistance causes some of the ball's kinetic energy to be transformed into heat, which increases the temperature of the air particles it interacts with, thus reducing the ball's final velocity compared to its initial velocity.
What is internal energy according to the script?
-Internal energy is the total energy contained within a system, including the kinetic energy of all the atoms or molecules, potential energy from bonds, and any other form of energy present.
How is the internal energy of an ideal gas simplified in the script?
-For an ideal gas, and even more so for a monoatomic ideal gas like helium or neon, the internal energy can be simplified to just the kinetic energy of the individual atoms, ignoring other factors like bond energy and potential energy.
What does the script state about the change in internal energy (ΔU)?
-The script states that the change in internal energy (ΔU) is equal to the heat added to the system (Q) minus the work done by the system (W), or alternatively, plus the work done on the system.
How does the script explain the concept of heat in relation to energy transfer?
-Heat is a form of energy transfer between systems or entities. When heat is added to a system, it increases the system's internal energy, and when heat is removed, it decreases the system's internal energy.
What is the relationship between work and energy according to the script?
-Work is a process of energy transfer. When work is done by a system, it loses energy, and when work is done on a system, it gains energy.
How does the script describe the transformation of kinetic energy into heat due to friction?
-The script describes that when an object, like the ball, experiences friction, it transfers its kinetic energy to the air particles it interacts with, causing them to vibrate faster and increasing their kinetic energy, which is perceived as heat.
What is the significance of the formula ΔU = Q - W or ΔU = Q + W as mentioned in the script?
-The formula ΔU = Q - W signifies that the change in internal energy is the result of the net energy transferred to or from the system through heat and work. The formula ΔU = Q + W is a less common representation but indicates that work done on the system results in an increase in internal energy, while work done by the system results in a decrease.
How does the script emphasize understanding the first law of thermodynamics?
-The script emphasizes understanding the first law of thermodynamics by providing concrete examples, such as the ball's energy transformation, and by explaining the concepts of internal energy, heat transfer, and work, reinforcing the idea that energy can only change forms and not be created or destroyed.
Outlines
🌟 Introduction to the First Law of Thermodynamics
The paragraph introduces the first law of thermodynamics, which states that energy cannot be created or destroyed, but only transformed from one form to another. The speaker uses the example of throwing a ball straight up to illustrate how kinetic energy is transformed into potential energy and back into kinetic energy. The concept is further explained with the consideration of air resistance, which converts some of the ball's kinetic energy into heat, demonstrating the conservation of energy according to the first law of thermodynamics.
🔄 Transformation of Kinetic and Potential Energy
This paragraph delves deeper into the transformation of kinetic and potential energy, using the example of a ball thrown upwards. The speaker explains how the kinetic energy of the ball is converted into potential energy at its peak and then back into kinetic energy as it falls. The presence of air resistance is considered, showing how it reduces the ball's final velocity due to the conversion of kinetic energy into heat, which is dissipated among air particles. The paragraph emphasizes the first law of thermodynamics and the concept of energy conservation.
🌡️ Understanding Internal Energy
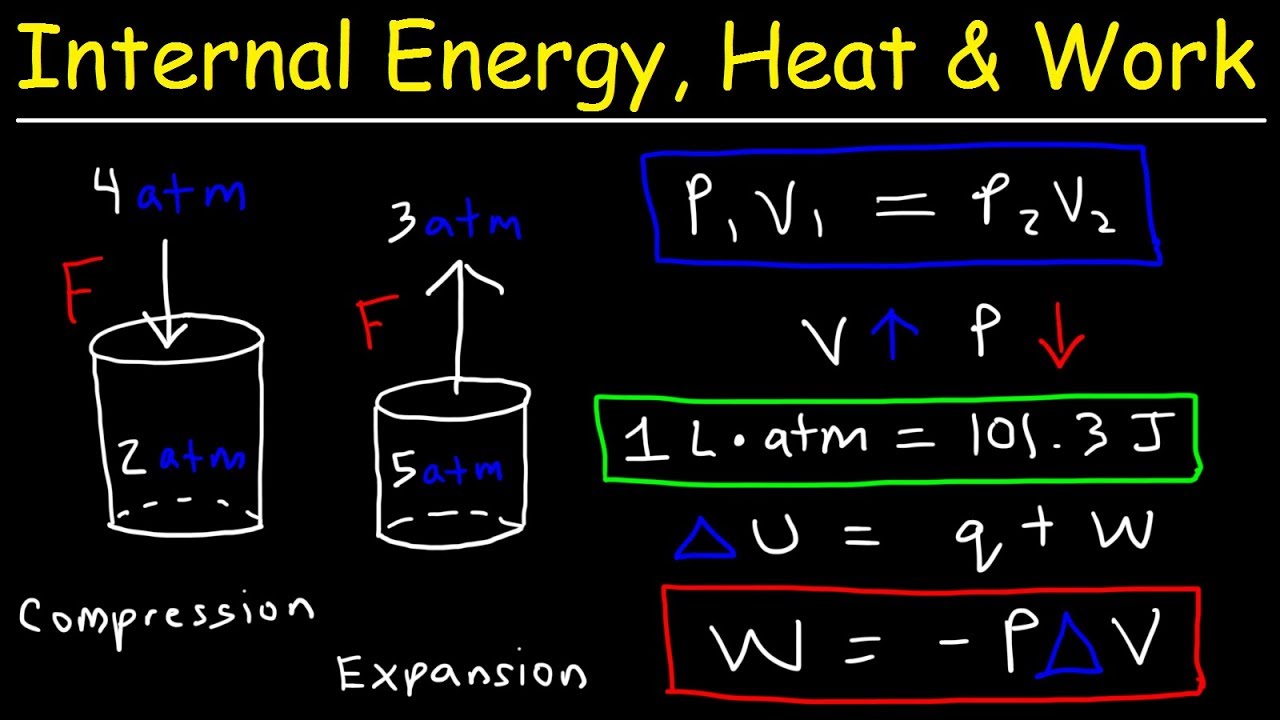
The speaker introduces the concept of internal energy, which is the total energy contained within a system. This includes the kinetic energy of all the atoms or molecules, potential energy from molecular vibrations, and other forms of energy present. The paragraph explains that internal energy is a macroscopic property that provides a broad description of the system's energy content. The speaker also discusses how to measure changes in internal energy, relating it to the first law of thermodynamics and highlighting that changes in internal energy are equal to the heat added to the system minus the work done by the system.
📈 Formulas and Notations for Energy Transfer
In the final paragraph, the speaker discusses various notations and formulas used to represent the change in internal energy. The first law of thermodynamics is used to express the change in internal energy (ΔU) as the heat added to the system (Q) minus the work done by the system (W). The speaker clarifies that when work is done by the system, energy is lost, and when heat is added, energy is gained. The paragraph concludes with the speaker promising to provide more examples and further explanation in the next video to help the audience fully understand the internal energy formula.
Mindmap
Keywords
💡Thermodynamics
💡First Law of Thermodynamics
💡Energy
💡Kinetic Energy
💡Potential Energy
💡Air Resistance
💡Friction
💡Internal Energy
💡Heat
💡Work
💡Transformation of Energy
Highlights
Introduction to the first law of thermodynamics, which states that energy cannot be created or destroyed, only transformed.
Explanation of energy transformation through the example of throwing a ball straight up in the air.
Discussion on the concept of kinetic and potential energy, and their interconversion.
Illustration of how air resistance affects the energy transformation of a ball thrown upwards.
Explanation of how kinetic energy is transformed into heat energy due to friction.
Introduction to the concept of internal energy as a macroscopic description of a system's energy content.
Explanation of how internal energy includes kinetic, potential, and other forms of energy in a system.
Clarification that for an ideal gas, internal energy can be simplified to represent only kinetic energy.
Formula for the change in internal energy (ΔU) in relation to heat (Q) and work (W) done by or on the system.
Discussion on the implications of the first law of thermodynamics on the change in internal energy.
Explanation of how work done by a system results in a loss of internal energy.
Clarification on the concept of heat transfer as a means of energy exchange between systems.
Different notations and expressions for the formula of change in internal energy.
Emphasis on the importance of understanding the direction of energy transfer in relation to work and heat.
Promise of further exploration of the internal energy formula in future videos.
Transcripts
Browse More Related Video

First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry

Enthalpy | Thermodynamics

More on internal energy | Thermodynamics | Physics | Khan Academy
First Law of Thermodynamics, Basic Introduction, Physics Problems
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

Energy Stores and Transfers
5.0 / 5 (0 votes)
Thanks for rating: