First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry
TLDRThis video explores the first law of thermodynamics, emphasizing the principle that energy cannot be created or destroyed, only transferred. It explains how internal energy changes through heat and work, using the analogy of money to illustrate energy transfer. The video distinguishes between open, closed, and isolated systems and clarifies the different perspectives in chemistry and physics regarding work done by or on a system. It also introduces the sign conventions for heat and work in the context of the first law, promising further practice in upcoming videos.
Takeaways
- 🔄 The first law of thermodynamics states that energy cannot be created or destroyed, only transferred from one place to another.
- ⚙️ There are two primary ways energy can be transferred: through heat and work.
- 🔍 The internal energy of a system, denoted by capital U, increases when heat flows into the system or when work is done on it.
- 📈 When the surroundings do work on a system, the system's internal energy increases, and the surroundings' energy decreases, illustrating the conservation of energy.
- 💡 The concept of energy transfer is analogous to money transfer, where one party's gain is another's loss, without creation or destruction.
- 🌐 Systems can be classified as open, closed, or isolated, each with different properties regarding the transfer of matter and energy.
- 🚫 In an open system, both matter and energy can be transferred, while a closed system allows only energy transfer, and an isolated system neither allows matter nor energy transfer.
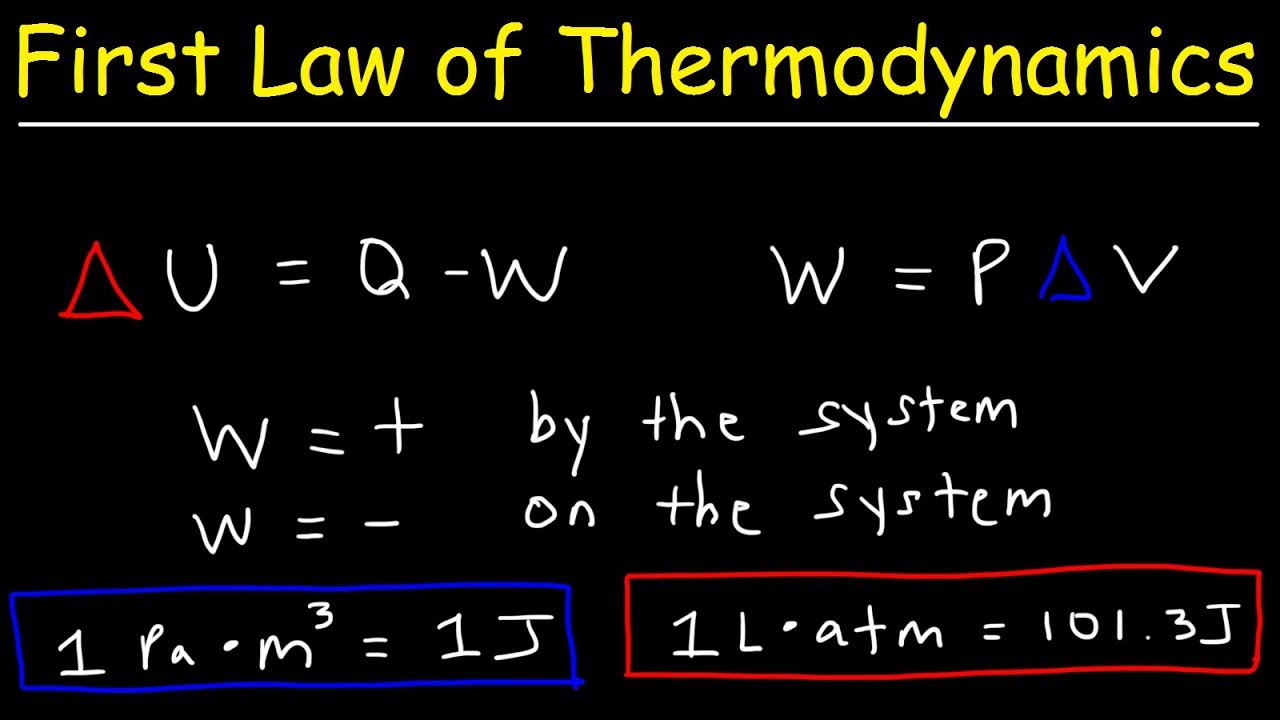
- 📚 The equation for the change in internal energy (ΔU) is Q + W, where Q is heat and W is work, commonly found in chemistry textbooks.
- 🔧 The physics perspective on the same equation is ΔU = Q - W, reflecting the viewpoint of the surroundings rather than the system.
- 🏋️ Work done by the system (W negative in chemistry) results in a decrease in internal energy as the system expends energy to perform work.
- 🔄 For an endothermic process, heat is absorbed by the system (Q positive), and for an exothermic process, heat is released by the system (Q negative).
- 📉 When work is done on the system (W positive in chemistry), the system's internal energy increases, whereas work done by the system decreases it.
Q & A
What is the basic idea behind the first law of thermodynamics?
-The first law of thermodynamics, also known as the law of conservation of energy, states that energy cannot be created or destroyed, but can only be transferred from one place to another.
How does the first law of thermodynamics relate to internal energy, heat, and work?
-The first law of thermodynamics relates to internal energy by stating that the change in internal energy of a system is equal to the heat added to the system plus the work done on the system, or the heat removed from the system minus the work done by the system.
What is the symbol used to represent internal energy in the script?
-The symbol used to represent internal energy in the script is the capital letter 'U'.
How does the script illustrate the concept of energy transfer using an example?
-The script uses the example of selling a laptop for $500 to illustrate the concept of energy transfer. Just as your bank account increases by $500 and the buyer's decreases by the same amount, energy is not created or destroyed but transferred.
What are the three types of systems mentioned in the script?
-The three types of systems mentioned are open systems, closed systems, and isolated systems.
How does the script differentiate between an open system and a closed system?
-An open system allows both matter and energy to be transferred into and out of it, while a closed system only allows energy to be transferred, not matter.
What is unique about an isolated system in the context of the first law of thermodynamics?
-An isolated system is unique because neither matter nor energy can enter or leave it. The mass and total energy within an isolated system remain constant.
What is the equation for the change in internal energy of a system according to the script?
-The equation for the change in internal energy of a system, according to the script, is ΔU = Q + W, where Q represents heat and W represents work.
Why is there a difference in the equation for the change in internal energy between chemistry and physics textbooks?
-The difference arises from the different perspectives taken in each field. Chemistry takes the system's point of view, while physics takes the surroundings' point of view, leading to different sign conventions for work done by or on the system.
What does the script mean by 'q is positive' and 'q is negative' in the context of heat energy?
-In the script, 'q is positive' means that the system is absorbing heat energy (endothermic process), and 'q is negative' means that the system is releasing heat energy (exothermic process).
How does the script define 'w is positive' and 'w is negative' in terms of work done by or on the system?
-The script defines 'w is positive' as work done on the system, which increases its internal energy, and 'w is negative' as work done by the system, which decreases its internal energy.
Outlines
🔄 First Law of Thermodynamics and Energy Transfer
This paragraph introduces the first law of thermodynamics, emphasizing the principle that energy cannot be created or destroyed, only transferred. It explains that energy can flow into or out of a system through heat and work, affecting the system's internal energy, denoted by the symbol 'U'. The paragraph uses the analogy of money to illustrate this concept, likening the transfer of energy to the transfer of money between bank accounts. It also outlines three types of systems: open, closed, and isolated, each with different properties regarding the transfer of matter and energy.
🔄 Equations and Perspectives on Energy Changes
The second paragraph delves into the mathematical representation of the first law of thermodynamics, highlighting the difference in perspective between chemistry and physics. In chemistry, the equation ΔU = q + w is used, where 'q' is the heat energy and 'w' is the work done by the system. Conversely, in physics, the equation ΔU = q - w is common, reflecting the viewpoint of the surroundings. The paragraph clarifies the sign conventions for 'q' and 'w': 'q' is positive for endothermic processes (heat absorbed) and negative for exothermic processes (heat released), while 'w' is negative when work is done by the system (decreasing internal energy) and positive when work is done on the system. The explanation aims to clarify these concepts for better understanding in practical applications.
🔥 Understanding Heat and Work in Thermodynamics
The final paragraph focuses on the concepts of endothermic and exothermic processes, as well as the sign conventions for heat (q) and work (w) in thermodynamics. It reiterates that 'q' is positive when the system absorbs heat and negative when it releases heat. Similarly, 'w' is positive when work is done on the system and negative when work is done by the system. The paragraph promises to provide practice problems in a subsequent video to help viewers calculate changes in internal energy using these principles.
Mindmap
Keywords
💡First Law of Thermodynamics
💡Internal Energy
💡Heat
💡Work
💡System
💡Surroundings
💡Open System
💡Closed System
💡Isolated System
💡Endothermic Process
💡Exothermic Process
Highlights
The first law of thermodynamics states that energy cannot be created or destroyed, only transferred.
Energy transfer occurs through heat and work, affecting a system's internal energy represented by the symbol capital U.
When heat flows into a system, it gains energy, increasing its internal energy.
The surroundings perform work on a system, which also increases the system's internal energy.
The energy gained by the system comes from the surroundings, illustrating the principle of energy conservation.
An analogy is made comparing energy transfer to money transactions, emphasizing no creation or destruction of energy or money.
Three types of systems are introduced: open, closed, and isolated, each with different properties for energy and matter transfer.
In an open system, both matter and energy can be transferred, unlike a closed system where only energy can be exchanged.
An isolated system does not allow energy or matter transfer, maintaining a constant total energy.
The equation for the change in internal energy is q + w, differing in perspective between chemistry and physics.
Chemistry views work done by the system as negative, while physics views it from the surroundings' perspective.
The sign conventions for q and w are crucial for understanding the direction of energy flow in thermodynamic processes.
An endothermic process is characterized by heat absorption, making q positive.
An exothermic process involves heat release, making q negative as the system loses energy to the surroundings.
Work done on the system increases its internal energy, making w positive in chemistry.
Work done by the system decreases its internal energy, making w negative from the system's perspective.
The video promises to provide practice problems on calculating changes in internal energy using heat and work in a future video.
Transcripts
Browse More Related Video
First Law of Thermodynamics, Basic Introduction, Physics Problems
First law of thermodynamics / internal energy | Thermodynamics | Physics | Khan Academy
Internal Energy, Heat, and Work Thermodynamics, Pressure & Volume, Chemistry Problems

GCSE Physics - Energy Stores, Transferring Energy & Work Done #1
6.1 Energy and the First Law of Thermodynamics | High School Chemistry
Thermodynamics and P-V Diagrams
5.0 / 5 (0 votes)
Thanks for rating: