Chemistry Science: Protons, Electrons & Neutrons Discovery
TLDRThis script narrates the historical journey of atomic discovery, starting with John Dalton's atomic theory in 1800. It describes J.J. Thomson's cathode ray tube experiment leading to the electron discovery, Ernest Rutherford's gold foil experiment revealing the atomic nucleus, and James Chadwick's identification of the neutron. The summary highlights the evolution of atomic structure understanding, from solid spheres to a nucleus with protons and neutrons, surrounded by orbiting electrons, and hints at the atomic structure's role in determining material properties.
Takeaways
- 🌌 In 1800, John Dalton proved the existence of atoms, which he believed to be the smallest particles in the universe, like solid spheres.
- 🔬 JJ Thompson discovered electrons in 1897 using a cathode ray tube, demonstrating that atoms contained even smaller particles with a negative charge.
- 🏆 Thompson's discovery of the electron earned him a Nobel Prize and he was known for helping his students become renowned scientists.
- 🎯 Ernest Rutherford, a student of Thompson, conducted the gold foil experiment, revealing that atoms are mostly empty space with a dense, positively charged nucleus.
- 💥 Rutherford's experiment showed that a small fraction of alpha particles were deflected or even bounced back, indicating a concentrated mass within the atom.
- 🚀 Rutherford's work led to the understanding that the atom's nucleus contains protons, which are responsible for its positive charge and atomic number.
- 🔍 The nucleus's mass was found to be greater than the sum of its protons, suggesting the presence of another particle, later identified as the neutron by James Chadwick.
- 📚 Chadwick discovered the neutron in 1932 by bombarding beryllium with alpha particles, which emitted uncharged particles of similar mass to protons.
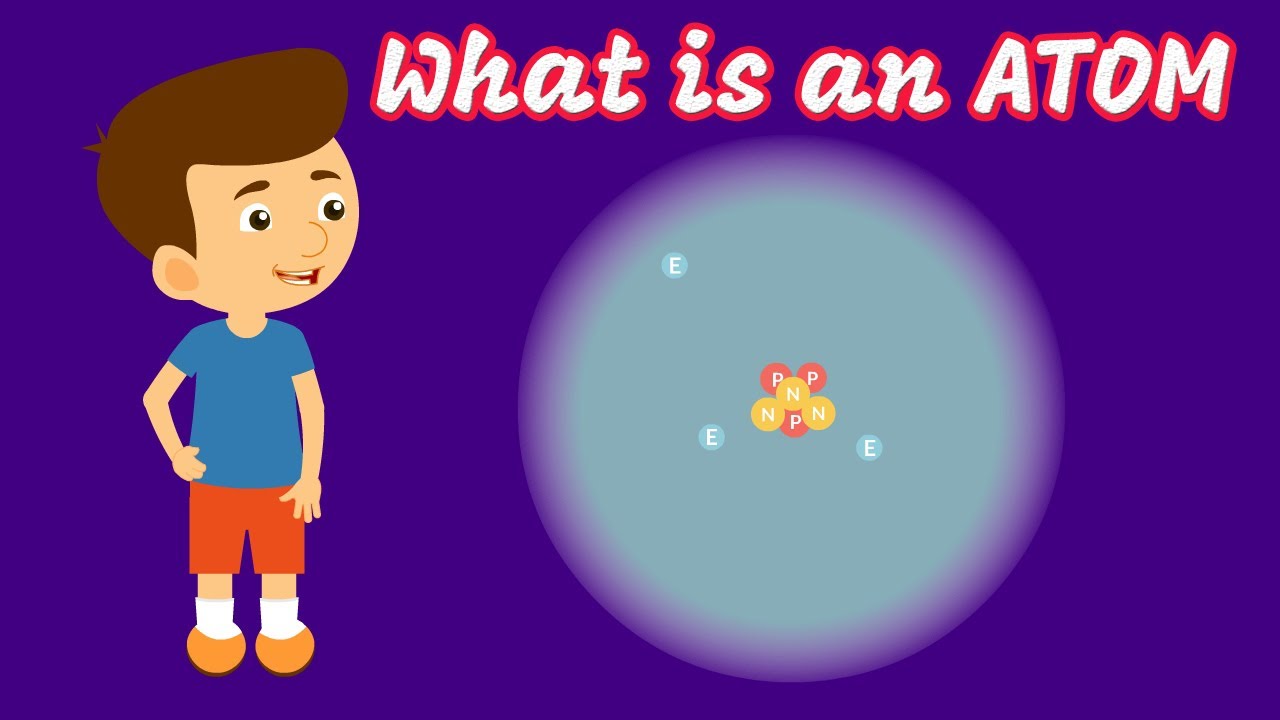
- 🔐 The atomic structure consists of a nucleus with protons and neutrons, and electrons orbiting at high speeds, held by powerful electric forces.
- 🔄 The electric forces binding atoms are vastly stronger than gravity, forming the basis of atomic stability and chemical interactions.
- 🌐 The arrangement of electrons in shells around the nucleus determines the properties of elements, such as state of matter, color, and metallic or non-metallic nature.
Q & A
Who was the scientist that proposed the idea of atoms in the 1800s?
-John Dalton was the scientist who proposed the idea of atoms in the 1800s, suggesting that matter was made of incredibly tiny building blocks called atoms.
What did Dalton initially believe about the structure of atoms?
-Dalton initially believed that atoms were solid spheres, similar to billiard balls, and that they were the smallest particles in the universe.
Who discovered the electron and won a Nobel Prize for this discovery?
-JJ Thompson discovered the electron and won a Nobel Prize for his discovery.
What apparatus did JJ Thompson use to discover the electron?
-JJ Thompson used a cathode ray tube, a glass tube filled with low-pressure gas and containing two metal plates, to discover the electron.
What is the significance of the cathode ray tube experiment?
-The cathode ray tube experiment was significant because it demonstrated that atoms contained even smaller particles, which were later identified as electrons.
Who was the student of JJ Thompson that later discovered the nucleus of the atom?
-Ernest Rutherford, a student of JJ Thompson, later discovered the nucleus of the atom.
What experiment did Rutherford conduct to discover the atom's nucleus?
-Rutherford conducted the gold foil experiment, firing alpha particles at a very thin sheet of gold to discover the atom's nucleus.
What did Rutherford's gold foil experiment reveal about the structure of atoms?
-Rutherford's gold foil experiment revealed that atoms were mostly empty space, with the mass concentrated in a very small, positively charged nucleus.
Who discovered the neutron and how did he discover it?
-James Chadwick discovered the neutron by shooting alpha particles into beryllium atoms and observing the particles that were knocked out of the beryllium nuclei.
What property of the neutron did Chadwick observe during his discovery?
-Chadwick observed that the newly discovered particles, later named neutrons, did not bend when passed through charged parallel plates, indicating they were electrically neutral.
How do the electric forces within an atom contribute to its structure and properties?
-The electric forces within an atom act as a kind of atomic glue, holding the negatively charged electrons in orbit around the positively charged nucleus. These forces dictate the properties of atoms, including their state of matter, color, brittleness, and whether they are a metal or nonmetal.
Outlines
🔬 Discovery of Subatomic Particles
The first paragraph introduces the concept of atoms as the fundamental building blocks of matter, as proposed by ancient Greeks and later proven by John Dalton. Dalton's model depicted atoms as solid spheres. However, the narrative shifts with the discovery of subatomic particles. J.J. Thomson's cathode ray tube experiment revealed electrons, which are lightweight and negatively charged, thus challenging Dalton's theory. Thomson's work led to the understanding that atoms are composed of smaller particles and earned him a Nobel Prize. The paragraph also highlights the influence of Thomson as a mentor to several notable scientists, including Ernest Rutherford, who contributed to atomic structure theories.
🌌 Rutherford's Gold Foil Experiment and Nucleus Discovery
The second paragraph delves into Rutherford's experiment with alpha particles and gold foil, which contradicted Thomson's model of a diffuse atomic mass. Rutherford observed that while most alpha particles passed through the foil, some were deflected or even bounced back, indicating a concentrated positive charge at the atom's center—the nucleus. This experiment led to the discovery of the atomic nucleus, which is small but contains most of the atom's mass. Rutherford's findings also introduced the concept of the proton as a component of the nucleus. The paragraph concludes with the introduction of the neutron by James Chadwick, which, along with protons, forms the nucleus, explaining the discrepancy in the atomic mass.
⚛️ Atomic Structure and Electron Orbitals
The final paragraph discusses the role of electric forces in holding atoms together, with electrons orbiting the nucleus due to their negative charge being attracted to the nucleus's positive charge. It emphasizes the immense strength of these electric forces compared to gravity. The paragraph also anticipates the next video, which will explore how electrons are arranged in shells around the nucleus, influencing the properties of elements such as state of matter, color, and metallic or non-metallic characteristics.
Mindmap
Keywords
💡Atoms
💡Cathode Ray Tube
💡Electrons
💡Alpha Particles
💡Atomic Nucleus
💡Protons
💡Neutrons
💡Scintillation Screen
💡Electric Forces
💡Atomic Number
💡Isotopes
Highlights
John Dalton proved the existence of atoms in 1800, theorizing them as solid spheres.
Atoms were later discovered to be composed of smaller particles.
JJ Thompson's cathode ray tube experiment revealed the presence of negatively charged particles, leading to the discovery of the electron.
Thompson's cathode ray tube demonstrated that electrons are much lighter than hydrogen atoms.
JJ Thompson's discovery of the electron earned him a Nobel Prize.
Ernest Rutherford conducted experiments with alpha particles, discovering the atom's nucleus.
Rutherford's gold foil experiment showed that atoms are mostly empty space with a dense nucleus.
The nucleus was found to be very small and incredibly heavy, containing over 99% of an atom's mass.
Rutherford discovered the proton, a particle inside the nucleus responsible for its positive charge.
James Chadwick discovered the neutron, an uncharged particle in the nucleus with similar mass to the proton.
Chadwick's discovery of the neutron was achieved by bombarding beryllium atoms with alpha particles.
The atomic nucleus consists of protons and neutrons, with electrons orbiting around it.
Electrons, with their negative charge, are attracted to the positively charged nucleus, held by electric forces.
Electric forces are significantly stronger than gravity, acting as the atomic glue.
The arrangement of electrons in shells around the nucleus dictates the properties of elements.
JJ Thompson was a mentor to several Nobel Prize-winning scientists, including his son.
Chadwick's life was marked by significant events, including being a prisoner of war and contributing to the atomic bomb's development.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: