The Basic Structure of the Atom | Chemistry and Our Universe: How it All Works
TLDRThe video script explores the structure of atoms, the smallest systems in the universe, and their composition of subatomic particles: protons, neutrons, and electrons. It traces the historical development of atomic theory from ancient Greek philosophers to John Dalton's law of conservation of mass and multiple proportions. The script delves into the discovery of the electron by JJ Thomson and Ernest Rutherford's gold foil experiment, leading to the nuclear model of the atom. It also discusses isotopes, atomic mass, and ions, highlighting the importance of electron configuration in determining elemental properties.
Takeaways
- 🌌 Nature's tendency to build larger structures from smaller components is evident in the universe, from galaxies to DNA.
- 🔬 Atoms are the smallest systems that follow the principle of being composed of smaller parts, and they can be further divided into subatomic particles.
- 💫 The ancient Greeks were the first to postulate the existence of atoms as fundamental, indivisible substances.
- 📜 John Dalton's atomic theory, based on the law of conservation of mass and the law of multiple proportions, revived the concept of atoms.
- 🔋 JJ Thomson's discovery of the electron and the plum pudding model marked the first attempts to explain the atomic structure.
- 💥 Ernest Rutherford's gold foil experiment disproved the plum pudding model, leading to the realization that atoms have a dense nucleus.
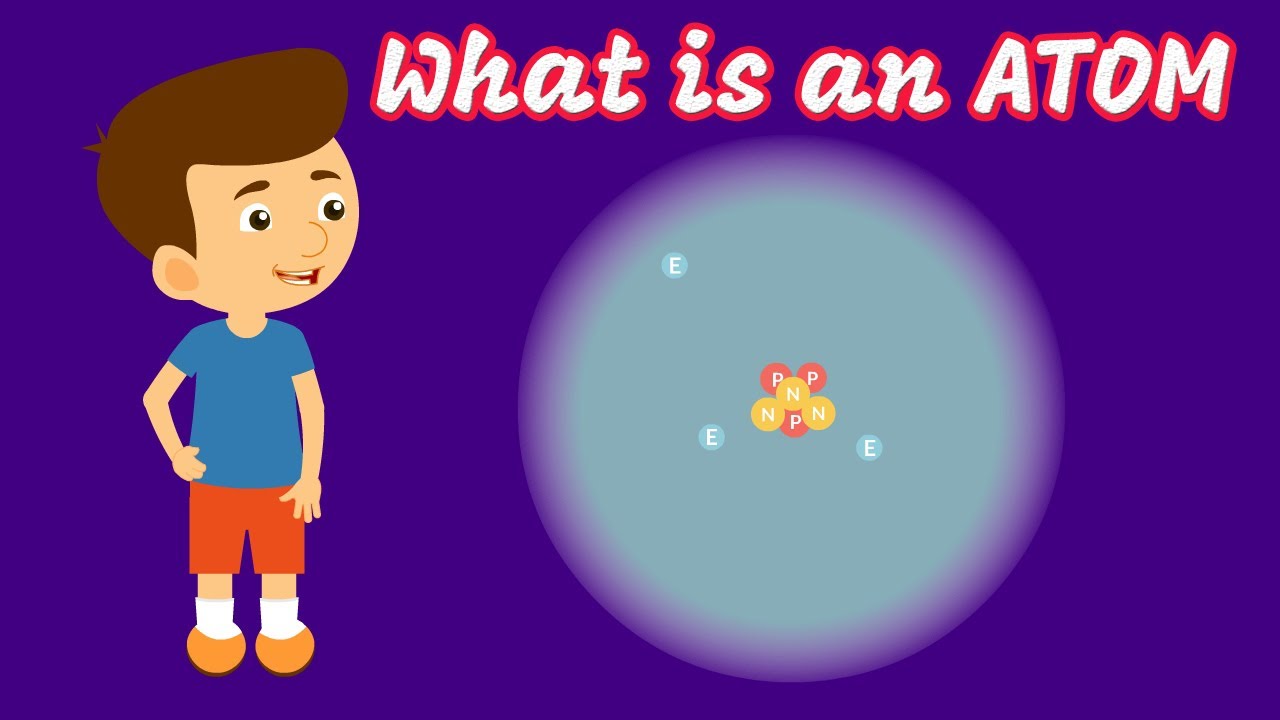
- 🎯 James Chadwick's discovery of the neutron completed the inventory of subatomic particles, consisting of protons, neutrons, and electrons.
- 🔩 The number of protons in an atom's nucleus defines its identity and is referred to as its atomic number.
- 📊 Isotopes are variants of an element with different numbers of neutrons, resulting in different atomic masses but similar chemical properties.
- 💡 Ions are charged atoms or molecules created by adding or removing electrons; anions are negatively charged while cations are positively charged.
Q & A
What is the significance of the structure principle discussed in the transcript?
-The structure principle discussed in the transcript highlights how nature uses a limited number of smaller components to create larger, complex structures, resulting in tremendous variation across different scales, from galaxies to biological systems. This principle is evident in the atomic structure, which is composed of subatomic particles.
What are the three types of subatomic particles that make up an atom?
-The three types of subatomic particles that make up an atom are protons, which carry a positive charge; electrons, which carry a negative charge; and neutrons, which have no charge.
How did John Dalton contribute to the atomic theory?
-John Dalton contributed to the atomic theory by formulating a sound atomic theory based on his observations. He combined the law of conservation of mass and the law of multiple proportions to argue for the existence of indivisible atoms that combine in simple whole-number ratios to create molecules.
What was the plum pudding model proposed by JJ Thomson?
-The plum pudding model proposed by JJ Thomson was an early atomic model where atoms were thought to consist of small negatively charged electrons embedded in a very low-density positively charged spherical matrix, resembling raisins in a bowl of pudding.
What experiment led Ernest Rutherford to disprove the plum pudding model?
-Ernest Rutherford conducted an experiment where he aimed a beam of alpha particles at a thin gold foil. The unexpected deflection of some alpha particles back towards the source indicated that the mass of gold atoms was concentrated in a small, dense nucleus, disproving the plum pudding model.
What did James Chadwick discover that led to the understanding of the neutron?
-James Chadwick discovered a new type of radiation that could penetrate other atomic nuclei without being repelled by the positive nuclei. This led him to deduce the existence of neutrons, uncharged particles that are slightly larger in mass than protons.
What are isotopes, and how do they differ from one another?
-Isotopes are groups of atoms of the same element that have the same number of protons but different numbers of neutrons, resulting in different atomic masses. They have very similar chemical properties but can vary significantly in mass.
How do ions differ from neutral atoms, and what are the two types of ions?
-Ions differ from neutral atoms in that they carry a net overall charge due to an imbalance in the number of protons and electrons. There are two types of ions: anions, which have a net negative charge due to excess electrons, and cations, which have a net positive charge due to a deficiency of electrons.
What is the significance of the atomic number, and how is it determined?
-The atomic number is significant because it defines the identity of an element. It is determined by the number of protons in the nucleus of an atom. Each element has a unique atomic number that corresponds to its position in the periodic table.
How does the structure of an atom influence the behavior of elements?
-The structure of an atom, particularly the arrangement of electrons in the electron cloud, is a key factor in determining how elements behave. The position of electrons within the electron cloud influences an element's chemical properties, reactivity, and bonding capabilities.
Outlines
🌌 The Ubiquity of Structure in the Universe
This paragraph introduces the concept of structure in the universe, highlighting how larger structures are composed of smaller components. It discusses the organization of stars into galaxies, planets into solar systems, and molecules into complex biological systems. The paragraph emphasizes the importance of understanding the atom, one of the smallest systems in creation, and sets the stage for a deeper exploration of atomic structure and its significance in the grand scheme of the cosmos.
💡 Dalton's Atomic Theory and the Law of Conservation of Mass
This section delves into John Dalton's atomic theory, which was based on observations and the law of conservation of mass. Dalton's work revolved around understanding chemical reactions, such as the formation of water and hydrogen peroxide from oxygen and hydrogen. His key insight was the law of multiple proportions, which revealed that elements combine in small whole-number ratios. These laws provided strong evidence for the existence of indivisible atoms, which were thought to be the fundamental units of matter.
🔬 Discovery of the Electron and the Plum Pudding Model
This paragraph discusses the discovery of the electron by J.J. Thomson through experiments with cathode rays. Thomson's work led to the proposal of the plum pudding model, which suggested that atoms consisted of a positively charged sphere with negatively charged electrons embedded within it. This model was a significant step towards understanding atomic structure, although it was later disproven by Thomson's own student, Ernest Rutherford.
💥 Rutherford's Gold Foil Experiment and the Nuclear Model
Ernest Rutherford's gold foil experiment is detailed here, which challenged and ultimately disproved the plum pudding model. Rutherford's findings indicated that atoms have a dense, positively charged nucleus with most of the atom's mass, surrounded by a cloud of light, negatively charged electrons. This led to the establishment of the nuclear model of the atom, which is a fundamental concept in modern atomic theory.
🔍 The Discovery of Isotopes and Atomic Mass
This section explains the concept of isotopes, which are atoms of the same element with different numbers of neutrons, resulting in varying atomic masses. The paragraph discusses how the number of protons defines an element's identity, while the number of neutrons can affect its mass. It also introduces the notation for isotopes and provides examples of how isotopes exist in nature, with different abundances and properties.
💫 The Complexity of Electron Clouds and Ions
The paragraph concludes by acknowledging that the electron cloud around an atom's nucleus is not uniform and contains a complex system of electron orbits. It also introduces the concept of ions, which are charged atoms formed by adding or removing electrons. The paragraph sets the stage for a deeper exploration of the electronic structure of atoms in the subsequent discussion.
Mindmap
Keywords
💡Structure
💡Subatomic Particles
💡Atom
💡John Dalton
💡JJ Thomson
💡Ernest Rutherford
💡James Chadwick
💡Isotopes
💡Ions
💡Atomic Number
💡Electronic Structure
Highlights
Nature's tendency to create larger structures from smaller components is a universal principle seen in everything from galaxies to DNA.
Atoms, one of the smallest systems in the universe, can be divided into smaller particles, changing their identity in the process.
Subatomic particles, protons, electrons, and neutrons, make up atoms and are fundamental to understanding the structure of matter.
The historical journey from ancient Greek philosophers to modern scientists has shaped our understanding of atoms.
John Dalton's atomic theory, based on the law of conservation of mass and the law of multiple proportions, was a significant step in atomic theory.
JJ Thomson's discovery of the electron and the plum pudding model marked the first attempt to explain the atomic structure.
Ernest Rutherford's gold foil experiment with alpha particles led to the realization that atoms have a dense nucleus, disproving Thomson's plum pudding model.
Rutherford's model, also known as the nuclear model, describes atoms as mostly empty space with a concentrated nucleus containing most of the atom's mass.
James Chadwick's discovery of the neutron completed the inventory of subatomic particles, showing that protons and neutrons contribute nearly all of an atom's mass.
The number of protons in an atom's nucleus determines its identity as an element, while the number of neutrons affects its mass, leading to isotopes.
Isotopes of an element have similar chemical properties but can differ in mass due to varying numbers of neutrons.
The periodic table reports an average atomic mass for elements that exist in multiple isotopes in nature.
Charged atoms, or ions, result from an imbalance between the number of protons and electrons, leading to anions or cations.
The electronic structure of an atom, including the complex system of electron orbits, is crucial in determining how elements behave.
The historical development of atomic theory and the discovery of subatomic particles have revolutionized our understanding of matter and the universe.
The atom's structure, with its dense nucleus and orbiting electrons, is a fundamental concept in chemistry and physics.
The study of atoms and their components has practical applications in various fields, including the development of new materials and technologies.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: