Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
TLDRThis educational script explores the concepts of ionization energy and electron affinity, using lithium as an example. It explains how ionization energy is the energy required to remove an electron from an atom, while electron affinity is the energy change when an electron is added. The script discusses the trends and exceptions in electron affinity across the periodic table, highlighting the complexity of this property compared to ionization energy.
Takeaways
- 🔬 Ionization energy is the energy required to remove an electron from a neutral atom, resulting in a positive ion.
- 🚀 Lithium, with an electron configuration of 1s² 2s¹, has an ionization energy that is positive, indicating energy is needed to remove its valence electron.
- ⚛️ Electron affinity is the energy change when an electron is added to a neutral atom, forming a negative ion.
- 📉 For lithium, electron affinity is negative, meaning energy is released when an electron is added, as seen with the electron configuration 1s² 2s².
- 🌐 The effective nuclear charge experienced by an electron is influenced by the number of shielding electrons, affecting both ionization energy and electron affinity.
- ❌ Neon, a noble gas with a full outer shell, has little to no electron affinity due to the complete shielding of the nucleus by its electrons.
- 📚 The electron affinity can be positive, as in the case of neon, where adding an electron requires energy rather than releasing it.
- 📉 Across a period in the periodic table, electron affinity generally increases due to the increasing effective nuclear charge attracting the added electron more strongly.
- ⚠️ There are exceptions to the trend of increasing electron affinity across a period, such as nitrogen, which does not follow the trend due to electron repulsion in its p orbitals.
- 📊 The most electronegative element, fluorine, has the highest electron affinity, releasing the most energy when an electron is added.
- 🔄 Going down a group in the periodic table shows inconsistencies in electron affinity trends, making it difficult to establish a general rule.
Q & A
What is ionization energy?
-Ionization energy is the energy required to remove an electron from a neutral atom, typically measured in kiloJoules per mol.
How does the electron configuration of a lithium atom differ from that of a lithium ion?
-A lithium atom has an electron configuration of 1s² 2s¹, while a lithium ion, formed by losing its valence electron, has a configuration of 1s² 2s⁰, resulting in a positive one charge.
Why is ionization energy positive?
-Ionization energy is positive because it takes energy to overcome the attractive force between the positively charged nucleus and the outermost electron to remove it from the atom.
What is electron affinity and how does it differ from ionization energy?
-Electron affinity is the energy change when an electron is added to a neutral atom. Unlike ionization energy, electron affinity can be negative, indicating energy is released when an electron is added.
Why does adding an electron to a lithium atom result in energy being released?
-Adding an electron to a lithium atom results in energy release because the added electron is attracted to the positively charged nucleus, leading to a negative electron affinity value.
How does the electron affinity of neon differ from that of lithium?
-Neon has a full outer shell with a stable electron configuration, making it unreactive and having little to no affinity for an additional electron. In contrast, lithium readily accepts an electron, showing a negative electron affinity.
What is the electron configuration of a neon atom and why is it unreactive?
-A neon atom has an electron configuration of 1s² 2s² 2p⁶, which is a stable, full outer shell configuration. It is unreactive because adding an electron would require energy due to the lack of attraction for the additional electron.
Why does the electron affinity trend increase as you move across a period in the periodic table?
-As you move across a period, the effective nuclear charge increases, which means the added electron feels a stronger pull from the nucleus, resulting in more energy being released and a higher electron affinity.
What is the exception to the increasing electron affinity trend as you move across a period?
-Nitrogen is an exception to the trend because adding an electron to its half-filled p orbital results in electron-electron repulsion, which does not follow the general trend of increasing electron affinity across a period.
Why does beryllium have a zero electron affinity value?
-Beryllium has a zero electron affinity value because adding an electron to its full 2s orbital requires energy, as the electron is effectively shielded from the nucleus and there is no net attraction for the additional electron.
How does the electron affinity of an element relate to its reactivity?
-Elements with high electron affinity, such as fluorine, are more reactive because they readily accept electrons to achieve a stable electron configuration. Conversely, elements with low or zero electron affinity, like neon, are less reactive.
Outlines
🔬 Ionization Energy and Electron Affinity Basics
The paragraph introduces the concepts of ionization energy and electron affinity using lithium as an example. It explains how ionization energy is the energy required to remove the valence electron from a neutral lithium atom, resulting in a lithium ion with a +1 charge. The process is depicted as energy-consuming, hence positive in value. Conversely, electron affinity is illustrated by adding an electron to lithium, which results in the release of energy, indicated by a negative value. The explanation emphasizes the attractive and repulsive forces acting on electrons and how these forces influence the energy changes during ionization and electron addition.
🌌 Electron Affinity Variations and Noble Gas Reactivity
This paragraph delves into the variations of electron affinity, particularly highlighting neon as an example of an atom with little to no affinity for an additional electron. The discussion centers on the effective nuclear charge experienced by an added electron and how it is completely shielded in the case of neon, leading to no energy release upon electron addition. The paragraph also touches on the electron affinity of other elements in the second period of the periodic table, noting exceptions to general trends and the unique case of beryllium, which requires energy to add an electron, thus having a zero or positive electron affinity value.
⬆️ Trends in Electron Affinity Across the Periodic Table
The final paragraph examines the trend of electron affinity as it increases across a period in the periodic table, culminating in fluorine having the highest electron affinity. It attributes this trend to the increasing effective nuclear charge, which results in a stronger attraction between the nucleus and the added electron, thus releasing more energy. The paragraph acknowledges exceptions to this trend, such as nitrogen, whose electron configuration and repulsion within its orbitals result in a lack of electron affinity, deviating from the observed pattern. The summary underscores the complexity of electron affinity compared to ionization energy and the limitations of general trends in explaining the behavior of all elements.
Mindmap
Keywords
💡Electron Affinity
💡Ionization Energy
💡Valence Electron
💡Shielding
💡Effective Nuclear Charge
💡Lithium Ion
💡Anion
💡Periodic Table
💡Noble Gas
💡Electron Configuration
💡Trend
Highlights
Review of ionization energy starting with a neutral lithium atom.
Explanation of lithium's electron configuration and its significance.
Concept of core electrons shielding the valence electron from the nucleus's positive charge.
Attraction between the nucleus and valence electron despite shielding.
Ionization energy as the energy required to remove the valence electron.
Introduction to electron affinity and its comparison with ionization energy.
Process of adding an electron to lithium and its impact on electron configuration.
Negative electron affinity value indicating energy release upon electron addition.
Comparison of lithium anion size to the neutral lithium atom.
Explanation of why noble gases like neon have zero electron affinity.
Effective nuclear charge and its role in electron affinity.
Trend of increasing electron affinity across a period in the periodic table.
Exceptions to the trend in electron affinity, such as beryllium and neon.
Explanation of why nitrogen does not follow the electron affinity trend.
General increase in electron affinity with increased effective nuclear charge.
Complexity of electron affinity compared to ionization energy and its exceptions.
Transcripts
Browse More Related Video

Periodic Trends of the Periodic Table

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character
Periodic Table Trends: Ionization Energy

Periodic Trends: Electron Affinity With Exceptions | Study Chemistry With Us
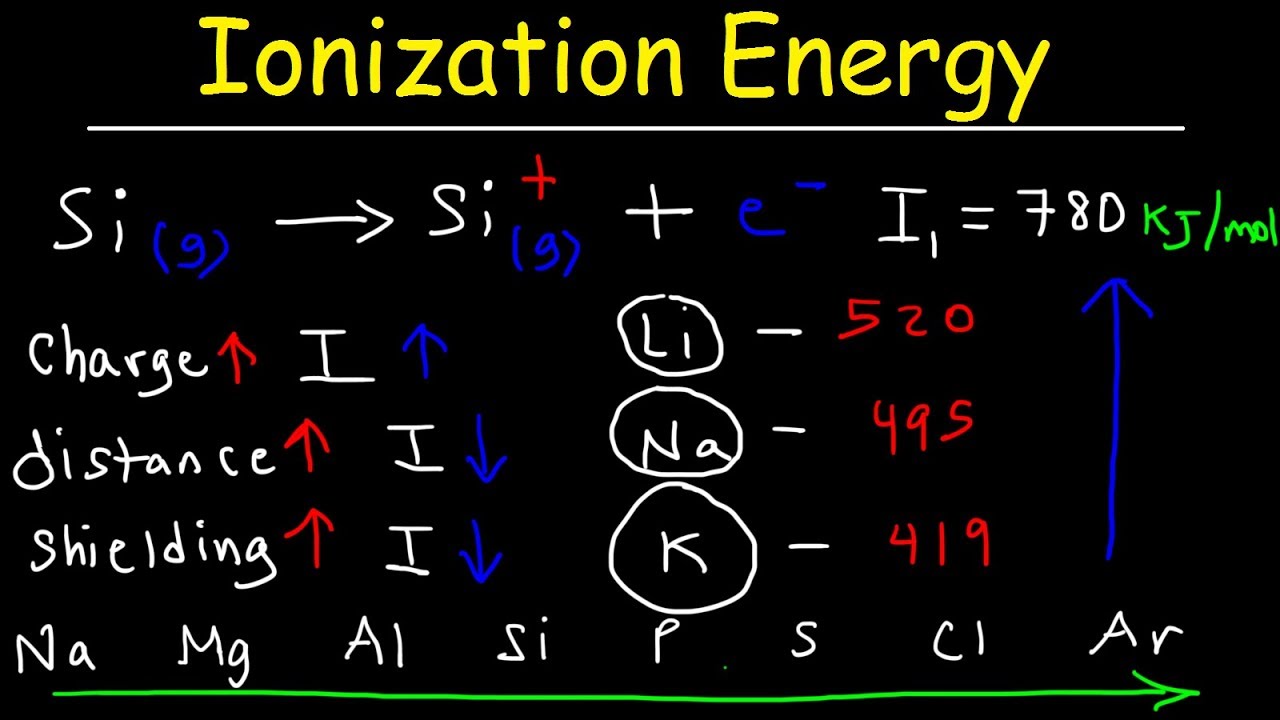
Ionization Energy - Basic Introduction

8. The Periodic Table and Periodic Trends
5.0 / 5 (0 votes)
Thanks for rating: