8. The Periodic Table and Periodic Trends
TLDRThis lecture delves into the intricacies of the periodic table, focusing on ionization energy and electron configuration. It explains the shift in energy levels between 4s and 3d orbitals and their implications on electron loss. Trends across the periodic table are explored, including the increase in ionization energy from left to right and the decrease from top to bottom. The lecture also touches on exceptions to these trends and introduces concepts like electron affinity and electronegativity, setting the stage for further discussion.
Takeaways
- 📚 The lecture discusses the significance of the periodic table and its arrangement based on electron configurations, emphasizing the importance of understanding these configurations for chemistry exams.
- 🔋 The concept of ionization energy is explained, highlighting its dependence on the effective nuclear charge and the trends in ionization energy across and down the periodic table.
- 🚀 The script addresses the '4s-3d' switch in energy levels when electrons are lost or gained, which affects the electron configuration and is a key point for understanding the behavior of elements.
- 🌟 A creative approach to learning about elements is introduced through music, using the song by 'They Might Be Giants' to illustrate the properties and uses of various elements.
- 💡 The periodic table is likened to an artist's paintbox, with each element being an ingredient used to create all materials, emphasizing chemistry's role in understanding the composition of everything.
- ⚛️ Trends in ionization energy are detailed, noting the increase across a period due to increasing effective nuclear charge and the decrease down a group due to increasing principal quantum number.
- 🔍 The script explains the concept of glitches in ionization energy trends, such as the anomaly with beryllium having a higher first ionization energy than boron, which is rationalized by the transition from 2s to 2p orbitals.
- 🔬 The method of photoelectron spectroscopy (PES) is introduced as a way to measure ionization energies by analyzing the kinetic energy of ejected electrons.
- 📉 The importance of understanding the quantum numbers n and l in determining ionization energies is discussed, as different orbitals within the same shell can have different ionization energies.
- 📚 The script provides insights into the historical context of the periodic table, explaining how its arrangement has evolved from properties to electron configurations.
- 🧲 The concept of electron affinity is introduced, explaining how it differs from ionization energy and the trends related to the gain of electrons by atoms, with halogens having the highest electron affinity and noble gases having negative electron affinities.
Q & A
What is the significance of the plus 1 in the electron configuration discussed in the script?
-The plus 1 indicates that an electron has been lost from the atom. This change in electron configuration affects the energy levels of the orbitals, particularly the 4s and 3d orbitals, which can switch energy levels when being filled.
Why do the 4s and 3d orbitals switch energy levels when being filled?
-The 4s and 3d orbitals switch energy levels due to the electron configuration changes that occur as electrons are added. This switch is significant because it affects which orbital loses an electron first and the overall stability of the ion.
How does the energy of the 4s and 3d orbitals relate to their electron configurations?
-The energy of the 4s and 3d orbitals is close, and they can switch levels depending on the number of electrons. This proximity in energy leads to the possibility of having half-filled or fully filled configurations, which can affect the atom's stability.
What is the importance of the periodic table in chemistry?
-The periodic table is crucial in chemistry as it organizes all the elements based on their properties and electron configurations. It serves as a foundation for understanding chemical reactions, bonding, and the properties of materials.
Why is the song by They Might Be Giants, 'Meet the Elements,' mentioned in the script?
-The song is used as a fun and engaging way to introduce the elements of the periodic table. It highlights the diverse roles elements play in various compounds and their importance in everyday life.
What is the concept of ionization energy in the context of the script?
-Ionization energy is the minimum energy required to remove an electron from an atom. The script discusses how ionization energy varies depending on the electron's orbital and the overall electron configuration of the atom.
How does the ionization energy of an atom change as you move across a period in the periodic table?
-As you move across a period, the ionization energy generally increases due to the increase in the effective nuclear charge (Z effective) while the principal quantum number (n) remains the same, leading to a stronger attraction between the nucleus and the electrons.
What is the significance of the first, second, and third ionization energies mentioned in the script?
-The first, second, and third ionization energies represent the energy required to remove the first, second, and third electrons from an atom, respectively. The script emphasizes that there can be significant differences in these values, indicating the varying stability of an atom as it loses electrons.
Why is the ionization energy different when removing an electron from boron, boron plus, and boron minus?
-The ionization energy differs due to the change in the shielding effect. When an electron is removed (boron plus), there is less shielding, leading to a higher effective nuclear charge and a stronger hold on the remaining electrons, thus requiring more energy to remove another electron.
What is the method used to measure ionization energies as discussed in the script?
-Photoelectron spectroscopy (PES) is the method used to measure ionization energies. It involves exciting an electron from an atom and measuring its kinetic energy. The ionization energy can then be calculated by subtracting the kinetic energy from the incident energy used to excite the electron.
What are electron affinities and how do they relate to the stability of an ion?
-Electron affinities refer to the energy change associated with an atom gaining an electron to form a negatively charged ion. A negative electron affinity indicates that energy is released and the resulting ion is more stable than the neutral atom, while a positive electron affinity suggests the ion is less stable.
Outlines
📚 Introduction to MIT OpenCourseWare and Electron Configuration
The script begins with an introduction to MIT OpenCourseWare, highlighting its mission to provide free, high-quality educational resources, with a call to support the initiative. The lecturer, Catherine Drennan, then delves into a chemistry lesson about electron configurations, explaining the significance of the 4s-3d energy switch when electrons are lost or gained, and how this affects the electron configuration of ions compared to neutral atoms. She emphasizes the importance of understanding these concepts for exam preparation and provides guidance on using old exams, noting that the material covered in the current lecture is typically on the first exam.
🎶 The Periodic Table's Role and Trends
The script transitions into a discussion about the periodic table, likening it to an artist's paintbox for chemists, containing all the elements that constitute everything in the world. The lecturer expresses excitement about teaching trends within the periodic table and hints at the importance of understanding electron configurations for exam success. A song by They Might Be Giants, 'Meet the Elements,' is introduced to illustrate the diverse properties and applications of elements, from their use in explosives to their necessity in the human body. The song is used as a fun and informative tool to emphasize the importance of learning about elements and their properties.
🔬 Ionization Energy and its Trends Across the Periodic Table
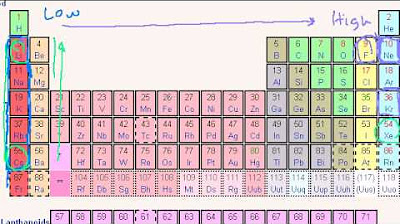
This section of the script focuses on ionization energy, defined as the minimum energy required to remove an electron from an atom. The lecturer explains how ionization energy is inversely related to binding energy and how it varies with different electron configurations within an atom. The concept of effective nuclear charge (Z effective) and its impact on ionization energy is discussed, with examples provided to illustrate the differences in ionization energy for various elements, such as boron, beryllium, and helium. The trends in ionization energy across periods and down groups in the periodic table are highlighted, with the underlying reasons for these trends being explained.
🚀 Differences in Ionization Energy and the Role of Electron Shielding
The script continues with a deeper exploration of ionization energy, emphasizing the differences in energy required to remove electrons from various orbitals within the same element, such as boron. The concept of electron shielding is introduced to explain why ionization energy varies between different ionic states of the same element, with a specific focus on the difference between neutral boron and boron plus. The lecturer uses a clicker question to engage students in understanding these concepts and provides an explanation for the observed trends in ionization energy.
🌐 General Trends and Anomalies in Ionization Energy
The lecturer discusses the general trends of ionization energy across the periodic table, noting the increase in ionization energy as you move from left to right across a period and the decrease as you move down a group. Anomalies or 'glitches' in these trends are also examined, such as the unexpected ionization energy of beryllium compared to boron and the slight increase for nitrogen compared to oxygen. The reasons behind these anomalies are explained, providing a deeper understanding of the periodic trends.
🔬 Measuring Ionization Energy Through Photoelectron Spectroscopy
This part of the script introduces the method of photoelectron spectroscopy (PES) as a means to measure ionization energies. The process involves using energy to excite an electron from an atom, such as neon, and then measuring the kinetic energy of the ejected electron. The relationship between incident energy, ionization energy, and kinetic energy is explained, and an example calculation is provided to demonstrate how ionization energies can be determined from the measured velocities of ejected electrons.
🧬 Electron Affinity and its Trends in the Periodic Table
The script concludes with an introduction to electron affinity, the ability of an atom to gain an electron and form a negatively charged ion. The concept is illustrated using chlorine as an example, which has a high electron affinity, indicating its eagerness to gain an electron and become more stable. The trends in electron affinity are discussed, with an increase in affinity as you move across a period and a decrease as you move down a group, culminating in the negative electron affinities of noble gases, which are stable and reluctant to gain additional electrons.
🔚 Conclusion and Preview of Upcoming Topics
In the final paragraph, the lecturer wraps up the discussion on electron affinity and hints at continuing the topic of electronegativity in the next lecture. The importance of understanding these concepts for the exams is reiterated, and the session ends with a clicker question to reinforce the understanding of electron affinities, particularly regarding noble gases.
Mindmap
Keywords
💡Ionization Energy
💡Electron Configuration
💡Periodic Table
💡Electron Affinity
💡Shielding Effect
💡Trends
💡Photoelectron Spectroscopy (PES)
💡Electronegativity
💡Valence Electrons
💡Noble Gases
💡Halogens
Highlights
Introduction to MIT OpenCourseWare and its mission to provide free educational resources.
Explanation of electron configuration and the significance of the 4s-3d energy switch.
Discussion on the differences between neutral atoms and ions, especially regarding the 4s-3d switch.
Importance of understanding electron configurations for exam preparation.
Clarification on the use of old exams for study and the changes in exam structure.
Introduction to the periodic table and its organization based on electron configurations.
The role of the periodic table in chemistry, likened to a painter's palette or a writer's vocabulary.
Engaging song by They Might Be Giants to illustrate the properties and uses of elements.
Differentiation between elements based on their reactivity and valence electrons.
The historical context of the periodic table's creation and its evolution.
Illustration of ionization energy and its relationship with atomic structure.
Discussion on the ionization energy trends across the periodic table.
Explanation of why ionization energy decreases down a group in the periodic table.
Analysis of anomalies in ionization energy trends and their rationalization.
Introduction to photoelectron spectroscopy as a method for measuring ionization energy.
Practical application of ionization energy concepts in understanding atomic structure.
Transition to the topic of electron affinity and its contrast with ionization energy.
Electronegativity as a related topic to be continued in the next lecture.
Transcripts
Browse More Related Video

Periodic Trends of the Periodic Table
Periodic Table Trends: Ionization Energy

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character

Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy

7.5 Periodic Trends | High School Chemistry

Periodic Trends - Atomic Radius, Electronegativity, Ionization Energy - Chemistry Series
5.0 / 5 (0 votes)
Thanks for rating: