Ionization Energy - Basic Introduction
TLDRThe video script provides an insightful introduction to ionization energy, the energy required to remove an electron from a gaseous atom or ion. Using silicon as an example, it explains how ionization energy increases with the positive charge of the ion, and how the electron's distance from the nucleus and shielding effects impact this energy. The script delves into silicon's electron configuration to illustrate why the second to third ionization energy jump is higher than the third to fourth, and emphasizes the significant increase when moving from a valence to a core electron. It also discusses periodic trends, noting how ionization energy generally increases across a period and decreases down a group, with exceptions due to sublevel differences and electron repulsion. The video concludes with examples comparing ionization energies of various elements and ions, reinforcing the principles outlined.
Takeaways
- 🔬 **Ionization Energy Definition**: Ionization energy is the energy required to remove an electron from a gaseous atom or ion, leading to ion formation.
- ⚡ **First Ionization Energy**: The energy needed to remove the first electron from an atom or ion, which can be different from subsequent ionization energies.
- 📈 **Increasing Ionization Energy**: As the charge of the ion increases, the ionization energy also increases, making it progressively harder to remove electrons.
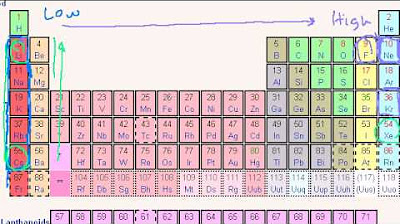
- 📊 **Ionization Energy Trends**: Ionization energy generally increases across a period (from left to right) and decreases down a group (from top to bottom) in the periodic table.
- 🛑 **Exceptions in Trends**: There are exceptions to the trend where ionization energy can decrease when moving from an s-block to a p-block or when moving from an unpaired to a paired electron.
- 🚀 **Electron Configuration Impact**: The ease of electron removal is influenced by the electron's distance from the nucleus and the shielding effect of inner electrons.
- ⚖️ **Nuclear Charge and Ionization Energy**: An increase in nuclear charge typically results in an increase in ionization energy, pulling electrons closer and making them harder to remove.
- 🛡️ **Shielding Effect**: The shielding effect reduces the ionization energy for electrons further from the nucleus that are shielded by inner electrons.
- 📉 **Decrease in Energy with Stability**: Electrons in more stable, lower energy orbitals are closer to the nucleus and harder to remove compared to those in higher energy orbitals.
- 🗺️ **Periodic Table and Ionization Energy**: The periodic table can be used to predict relative ionization energies of elements based on their position, considering trends and exceptions.
- 🔑 **Identifying Unknown Elements**: Large jumps in ionization energy values can indicate the number of valence electrons and help identify unknown elements based on their position in the periodic table.
Q & A
What is ionization energy?
-Ionization energy is the energy required to remove an electron from a gaseous atom or ion, resulting in its ionization.
How does the ionization energy of silicon compare to its ionized state?
-The ionization energy of silicon is 780 kilojoules per mole for the first electron removal. As more electrons are removed and the charge on the silicon ion increases, the ionization energy also increases.
Why does the ionization energy increase as you move from the first to the second ionization energy of silicon?
-The ionization energy increases from the first to the second ionization energy because the second electron removal involves an electron that is closer to the nucleus and more shielded by other electrons, making it harder to remove.
What is the significance of the jump in ionization energy from the second to the third ionization energy of silicon?
-The jump in ionization energy from the second to the third ionization energy is significant because it involves the removal of a 3s electron, which is closer to the nucleus and more shielded than the 3p electron removed in the second ionization energy.
How does the electron configuration of silicon influence its ionization energy?
-The electron configuration of silicon (1s² 2s² 2p⁶ 3s² 3p²) influences its ionization energy by determining which electrons are removed during ionization. The 3p electrons are removed first, followed by the 3s, and then the 2p, with each successive removal requiring more energy due to increased nuclear charge and decreased shielding.
Why is the jump from the fourth to the fifth ionization energy of silicon so large?
-The jump from the fourth to the fifth ionization energy is large because it involves the removal of a core electron (2p electron), which is closer to the nucleus and more tightly bound than the valence electrons in the higher energy levels.
How do the factors of nuclear charge, electron distance, and shielding affect ionization energy?
-Nuclear charge, electron distance, and shielding all affect ionization energy. As the nuclear charge increases, the ionization energy generally increases. The further an electron is from the nucleus, the lower its ionization energy due to weaker attraction. More shielding from inner electrons reduces the effective nuclear charge felt by outer electrons, thus lowering the ionization energy.
What is the general trend of ionization energy across the periodic table?
-The general trend of ionization energy across the periodic table is that it increases as you move up a group and towards the right across a period.
Why does the ionization energy of sodium (Na) differ from that of lithium (Li), even though sodium has a higher nuclear charge?
-The ionization energy of sodium is lower than that of lithium due to the greater distance of the valence electron from the nucleus in sodium and the increased shielding effect of the inner electrons. These factors outweigh the effect of the higher nuclear charge in sodium.
What causes the discontinuity in ionization energy when moving from magnesium (Mg) to aluminum (Al) in the periodic table?
-The discontinuity is caused by the change in sublevel from 3s in magnesium to 3p in aluminum. The 3p sublevel is further from the nucleus and more shielded, resulting in a lower ionization energy for aluminum compared to magnesium.
How does electron-electron repulsion affect the ionization energy of elements?
-Electron-electron repulsion affects ionization energy because unpaired electrons are in a more stable and lower energy state than paired electrons. This means that it generally requires less energy to remove a paired electron than an unpaired electron, leading to a lower ionization energy for elements with paired valence electrons.
Outlines
🔬 Introduction to Ionization Energy
This paragraph introduces ionization energy as the energy required to remove an electron from a gaseous atom, using silicon as an example. It explains the concept of ionization and how it leads to the formation of ions. The first ionization energy for silicon is given as 780 kJ/mol, and subsequent ionization energies are detailed, showing a pattern of increasing difficulty and energy required to remove each successive electron. The relationship between the charge of the ion and ionization energy is discussed, with the observation that ionization energy increases with the charge of the ion.
📈 Trends in Ionization Energy
The second paragraph delves into the reasons behind the increasing ionization energy as electrons are removed from an atom. It discusses the electron configuration of silicon and how the removal of different types of electrons (3s versus 3p) affects the ionization energy. The concept of shielding is introduced, explaining that core electrons shield valence electrons from the nucleus, making it harder to remove core electrons. The paragraph also highlights the significant increase in ionization energy when moving from the removal of a valence electron to a core electron, as seen when comparing the fourth and fifth ionization energies of silicon.
🔽 Periodic Trends of Ionization Energy
This section examines the periodic trends in ionization energy, noting that ionization energy generally increases across a period and decreases down a group in the periodic table. Using lithium, sodium, and potassium as examples, the paragraph illustrates how ionization energy changes within the same group of elements. It also explains the factors contributing to these trends, including the nuclear charge, the distance of the electron from the nucleus, and the shielding effect. The exceptions to these trends, such as the decrease in ionization energy when moving from magnesium to aluminum, are also discussed.
⚛️ Electron Repulsion and Ionization Energy
The fourth paragraph explores the concept of electron repulsion and its effect on ionization energy. It explains that electrons prefer to be unpaired and that paired electrons are less stable and easier to remove than unpaired electrons. This principle is used to understand why certain elements have lower ionization energies than their neighboring elements in the periodic table. The paragraph also uses the example of phosphorus and sulfur to illustrate this concept, showing how the stability of an electron configuration can influence ionization energy.
🏆 Comparing Ionization Energies
This paragraph presents a series of comparisons between different elements and ions to determine which has the higher ionization energy. It discusses the general trend that ionization energy increases as you move up and to the right in the periodic table but also notes exceptions due to sublevel transitions (e.g., from s to p sublevels) and electron pairing. Specific examples given include comparisons between magnesium and calcium, silicon and phosphorus, beryllium and boron, nitrogen and oxygen, and arsenic and sulfur, with an explanation of why each element has the ionization energy it does.
📊 Ranking Elements by Ionization Energy
The sixth paragraph involves ranking a series of elements by their first ionization energy. It provides a method for determining the order by considering their position in the periodic table and the trends in ionization energy. The elements barium, iron, silicon, oxygen, and helium are arranged in order of increasing first ionization energy, with helium having the highest due to its position towards the top right of the periodic table. The paragraph also includes actual ionization energy values for each element to illustrate the trend.
🔍 Identifying Unknown Elements by Ionization Energy
The final paragraph discusses how to identify an unknown element based on its ionization energy data, particularly focusing on the jump between ionization energies. A large jump between the third and fourth ionization energies indicates that the element has three valence electrons, leading to the identification of aluminum as the unknown element. The paragraph explains how to apply this method to other elements and ionization energy data, using potassium, aluminum, sulfur, krypton, or silicon as examples.
Mindmap
Keywords
💡Ionization Energy
💡First Ionization Energy
💡Second Ionization Energy
💡Electron Configuration
💡Valence Electrons
💡Core Electrons
💡Nuclear Charge
💡Shielding Effect
💡Periodic Trends
💡Electron Repulsion
💡Ion
Highlights
Ionization energy is defined as the energy required to remove an electron from a gaseous atom, turning it into an ion.
Silicon's first ionization energy is 780 kilojoules per mole, and its second ionization energy is 1575 kilojoules per mole.
As the charge of a gaseous atom or ion increases, the ionization energy also increases.
The jump from the second to the third ionization energy is higher due to the removal of a 3s electron, which is closer to the nucleus and more shielded than a 3p electron.
The fifth ionization energy of silicon is significantly higher because it involves removing a core electron from the 2p level.
Factors affecting ionization energy include nuclear charge, the distance of the electron from the nucleus, and shielding effects.
Ionization energy generally increases across a period from left to right on the periodic table and decreases as you go down a group.
The ionization energy of sodium is lower than lithium's due to increased shielding and greater distance of the valence electron from the nucleus.
An exception to the trend of increasing ionization energy from left to right on the periodic table occurs when moving from the s block to the p block.
Electron-electron repulsion causes paired electrons to be more easily removed than unpaired electrons, as seen in the ionization energy difference between phosphorus and sulfur.
Magnesium has a higher ionization energy than calcium because it is higher up in the periodic table.
Phosphorus has a higher ionization energy than silicon due to its position to the right of silicon on the periodic table.
Beryllium has a higher first ionization energy than boron, despite being to the left, because it is in the 2s sublevel compared to boron's 2p sublevel.
Nitrogen has a higher ionization energy than oxygen due to the stability of its unpaired 2p electron.
Sulfur has a higher ionization energy than arsenic following the trend of increasing energy as you move up and to the right on the periodic table.
The ionization energy of an ion increases with its positive charge, and ions with a higher positive charge or less negative charge have a higher ionization energy.
To determine the identity of an unknown element from ionization energy data, look for significant jumps in ionization energy values which indicate the removal of core electrons.
Aluminum is identified as the unknown element with three valence electrons based on a significant jump in the fourth ionization energy.
Transcripts
Browse More Related Video

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character

Periodic Trends of the Periodic Table

Periodic Trends: Ionization Energy Explained With Exceptions | Study Chemistry With Us

Trends in the Periodic Table
Periodic Table Trends: Ionization Energy

Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: