Second Law of Thermodynamics - Heat Energy, Entropy & Spontaneous Processes
TLDRThis video explores the second law of thermodynamics, focusing on heat energy and entropy. It explains that heat naturally flows from hot to cold, not the reverse, without external energy input. The video uses the analogy of a ball rolling downhill to illustrate this spontaneous process, which leads to an increase in entropy, signifying a move towards disorder. It emphasizes that natural systems, like a messy room or aging car, tend to degrade rather than improve without effort, highlighting the law's principle that spontaneous processes result in higher disorder.
Takeaways
- 🔥 The second law of thermodynamics states that heat naturally flows from a hot object to a cold one, not the other way around.
- 🔧 To reverse this flow, such as in a refrigerator, energy must be input into the system to pump heat out.
- ⛰️ The analogy of a ball rolling downhill illustrates the spontaneous flow of heat from higher to lower temperatures.
- 🏠 Entropy is a measure of disorder, and for natural processes, it tends to increase, indicating a move towards greater disorder.
- 🔄 In an ideal reversible process, entropy can remain at zero, but most real-world processes result in increased entropy.
- 📈 The increase in entropy is a direct consequence of natural processes, leading to higher disorder over time.
- 🧹 Effort is required to maintain order, as natural systems, such as a room, tend to become messy without intervention.
- 🚗 Degradation is a natural process; objects like cars left outside will deteriorate rather than improve over time.
- 💔 The spontaneous breaking of a glass cup represents the irreversible increase in disorder, as pieces do not reassemble on their own.
- 🔄 The second law of thermodynamics highlights the universal tendency of systems to move from order to disorder without external energy input.
- 🌐 This principle is fundamental to understanding energy transformations and the directionality of natural processes in the universe.
Q & A
What is the second law of thermodynamics?
-The second law of thermodynamics states that heat naturally flows from a higher temperature to a lower temperature and that natural processes tend to increase the overall entropy of the system.
Why does heat not flow from cold to hot without external influence?
-Heat does not flow from cold to hot without external influence because it would violate the second law of thermodynamics, which dictates that natural processes lead to an increase in entropy.
How does a refrigerator exemplify the second law of thermodynamics?
-A refrigerator exemplifies the second law by pumping heat from the cooler interior to the warmer exterior, which requires energy input, demonstrating that heat does not naturally flow from cold to hot.
What is entropy and how is it related to natural processes?
-Entropy is a measure of disorder in a system. In natural processes, the change in entropy is greater than zero, indicating an increase in disorder over time.
Can entropy decrease in a natural process?
-In a natural process, entropy cannot decrease because it would violate the second law of thermodynamics, which states that natural processes lead to an increase in entropy.
What is an ideal reversible process in the context of thermodynamics?
-An ideal reversible process is a theoretical process in which the entropy remains constant. It is a hypothetical scenario where no energy is lost and the system can be perfectly reversed to its initial state.
How does the second law of thermodynamics relate to the concept of energy conservation?
-The second law of thermodynamics complements the law of energy conservation by indicating that while energy is conserved, its quality degrades over time as systems tend towards increased disorder.
Why does it take effort to clean a room, according to the second law of thermodynamics?
-Cleaning a room requires effort because, according to the second law, natural systems tend towards disorder. Organizing a room is an act against this natural tendency, thus requiring energy input.
What is an example of a natural process that increases entropy?
-An example of a natural process that increases entropy is the rusting of a car left outdoors, where the car naturally deteriorates over time, increasing its disorder.
How does the breaking of a glass cup illustrate the second law of thermodynamics?
-The breaking of a glass cup illustrates the second law by showing that the pieces do not naturally reassemble, indicating that natural processes lead to an increase in disorder rather than a decrease.
What does it mean for a system to be in a state of disorder?
-A system in a state of disorder means that its components are randomly arranged with no specific order or pattern, which corresponds to a higher entropy state.
Outlines
🔥 Second Law of Thermodynamics: Heat Flow and Entropy
This paragraph introduces the second law of thermodynamics, focusing on heat energy and entropy. It explains that heat naturally flows from a hot object to a cold one, a spontaneous process that does not require external energy. The law is illustrated with the analogy of a ball rolling downhill, emphasizing that reversing this flow, such as in a refrigerator, requires energy input. The paragraph also touches on the concept of entropy, stating that for spontaneous processes, entropy increases, indicating a move towards disorder. It concludes with examples from everyday life, such as the natural tendency of rooms to become messy and objects to deteriorate over time, to illustrate the universal principle of systems moving towards a state of greater disorder.
Mindmap
Keywords
💡Second Law of Thermodynamics
💡Heat Energy
💡Entropy
💡Spontaneous Process
💡Reversible Process
💡Disorder
💡Energy Input
💡High Temperature
💡Low Temperature
💡Natural Systems
💡Orderly State
Highlights
Heat naturally flows from hot to cold objects without external energy input.
Heat does not spontaneously flow from cold to hot; energy must be pumped into the system.
Refrigerators exemplify the principle of pumping heat energy out, requiring external energy.
The analogy of a ball rolling downhill illustrates heat's natural flow from high to low temperature.
Heat cannot naturally flow uphill without energy input, similar to cold to hot heat transfer.
Heat pumps can transfer heat to warm environments, but at an energy cost.
Spontaneous processes, such as heat flow from hot to cold, result in increased entropy.
The second law of thermodynamics states that natural processes lead to greater entropy.
In ideal reversible processes, entropy can be zero, but most real systems are not perfectly reversible.
Natural spontaneous processes tend to increase disorder, as entropy is generally positive.
Maintaining order, like cleaning a room, requires energy and effort against natural disorder.
Natural systems, like aging houses or cars, degrade over time without external maintenance.
The second law of thermodynamics implies that systems tend towards a state of increased disorder.
Orderly states, like a whole glass cup, require energy to repair from a disordered state.
The difficulty of converting disordered states to ordered ones without energy input is highlighted.
The general idea behind the second law of thermodynamics is the tendency of systems to increase entropy.
Transcripts
Browse More Related Video

Entropy - 2nd Law of Thermodynamics - Enthalpy & Microstates

Entropy: Embrace the Chaos! Crash Course Chemistry #20
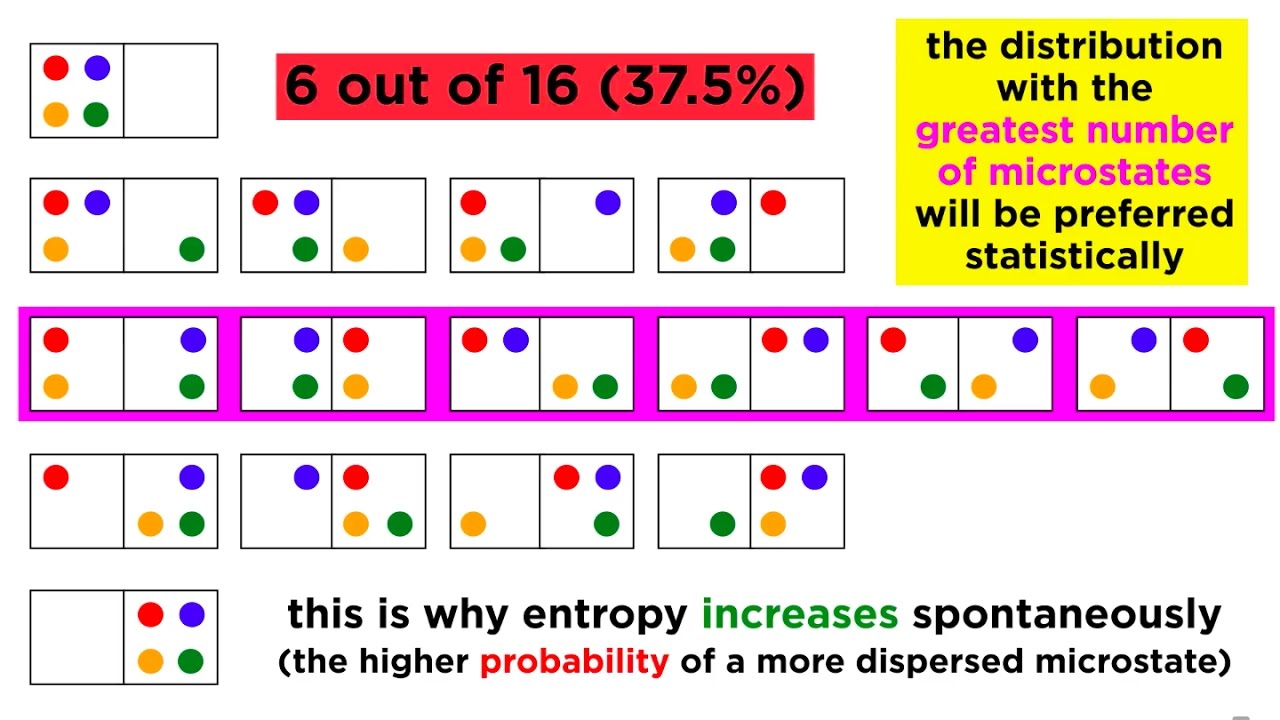
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates
24. The Second Law of Thermodynamics (cont.) and Entropy
What is entropy? - Jeff Phillips

6.2 Entropy, Gibbs Free Energy, and the Equilibrium Constant | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: