The Bohr Atom
TLDRThe video script from Mr. Andersen's AP Physics essential video 4 delves into the atomic structure and the Bohr model. It begins with Rutherford's discovery of the atomic nucleus and the unresolved issue of electron behavior. Niels Bohr identified a flaw in Rutherford's planetary model, which suggested electrons would spiral into the nucleus due to energy loss from electromagnetic radiation. Bohr proposed that electrons occupy quantized energy levels and can only move between these levels by absorbing or emitting photons, which explains the discrete spectral lines observed in atomic spectra. The Bohr model, particularly applicable to hydrogen, introduced the concept of quantization and helped to describe the atomic spectra, contributing to our understanding of atomic structure. The video also touches on the significance of electrons in determining an atom's properties and the construction of the periodic table.
Takeaways
- 🧲 Ernest Rutherford's gold foil experiment revealed a small positive nucleus at the center of an atom.
- 🌌 Niels Bohr identified a flaw in Rutherford's planetary model, noting that electrons would lose energy and spiral into the nucleus if they continuously emitted radiation.
- 🌈 Bohr's model introduced the concept of quantized energy levels, where electrons can only exist at specific levels and not in between.
- 🔋 Electrons move between these energy levels by absorbing or emitting photons, which helped explain the observed atomic spectra.
- 💡 The atomic spectra observed were not smooth but discrete, which contradicted the idea of continuous energy loss and required a new model to describe them.
- 📊 Bohr's model was particularly successful in explaining the hydrogen atom's spectral lines and energy level transitions.
- 🔬 The atomic number (number on the periodic table) indicates the number of protons in an atom's nucleus.
- ⚛️ In a neutral atom, the number of protons equals the number of electrons, which are crucial for determining an atom's chemical properties.
- 🌐 The electrons are found in a cloud around the nucleus, while protons and neutrons are located within the nucleus.
- 🔑 Bohr's model helped to refine the understanding of atomic structure by illustrating the quantized movement and energy states of electrons.
- 🚀 The Bohr model, while a significant advancement, is limited in its applicability and serves as a foundational step towards a more comprehensive atomic theory.
Q & A
What significant discovery did Ernest Rutherford's gold foil experiment contribute to atomic theory?
-Rutherford's gold foil experiment led to the discovery of a small, positively charged nucleus at the center of an atom.
What problem did Niels Bohr identify with Rutherford's planetary model of the atom?
-Bohr recognized that according to classical physics, electrons moving in orbits around the nucleus would emit electromagnetic radiation, lose energy, and spiral into the nucleus, causing the atom to collapse.
How does Bohr's model address the issue of electrons emitting electromagnetic radiation?
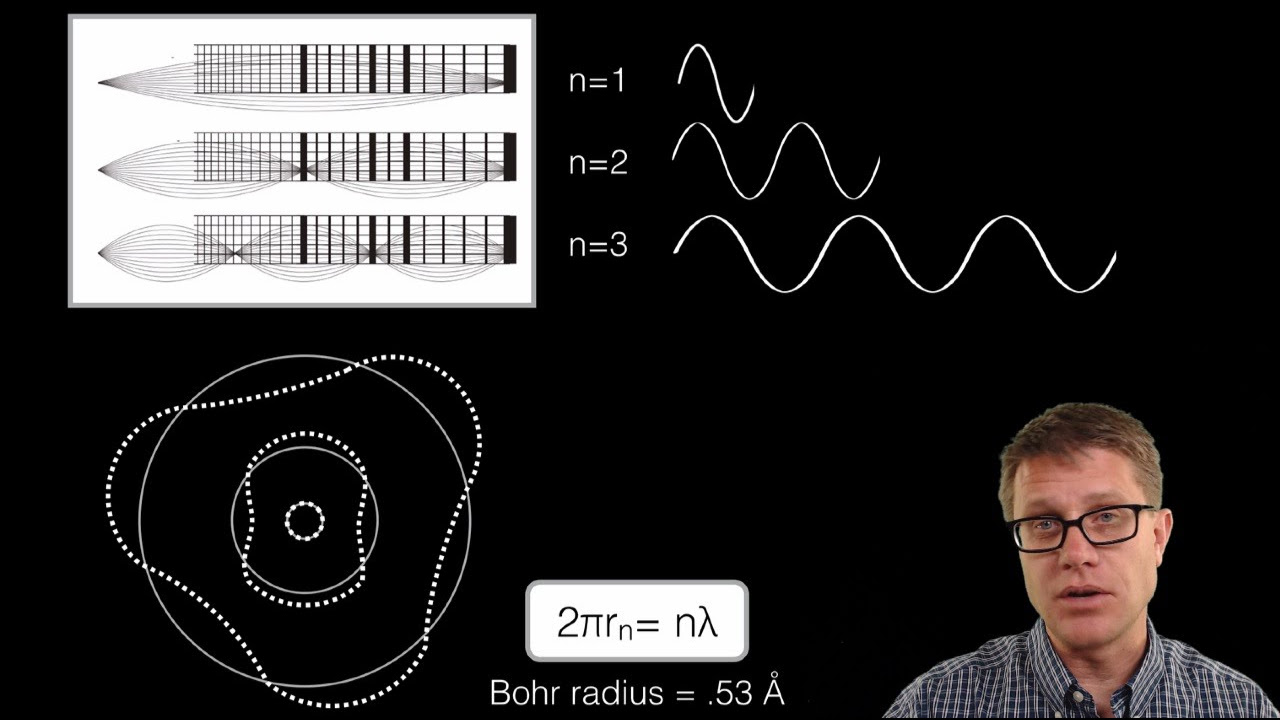
-Bohr's model introduced the concept of quantized energy levels, where electrons can only exist at certain discrete energy levels and do not emit radiation while in these states.
What observation about light spectra did Bohr's model help to explain?
-Bohr's model helped to explain the discrete lines observed in atomic spectra, rather than a continuous spectrum that would be expected if electrons emitted radiation while moving in orbits.
How does an electron move between energy levels according to Bohr's model?
-An electron moves between energy levels by absorbing a photon to jump to a higher level or by emitting a photon when it drops to a lower level.
What is the significance of the quantization of energy levels in Bohr's model?
-Quantization of energy levels means that electrons can only occupy specific, discrete energy states, which helps to explain the observed line spectra of atoms.
How does the Bohr model describe the structure of the atom?
-The Bohr model describes the atom as having a nucleus containing protons and neutrons, with electrons moving in quantized orbits around the nucleus.
What is the relationship between the number of protons and electrons in a neutral atom?
-In a neutral atom, the number of protons is equal to the number of electrons, balancing the positive and negative charges.
How does the atomic number relate to the number of protons in an atom?
-The atomic number represents the number of protons in the nucleus of an atom.
What is the mass number and how can it be used to estimate the number of neutrons in an atom?
-The mass number is the total number of protons and neutrons in an atom's nucleus. The number of neutrons can be estimated by subtracting the atomic number (number of protons) from the mass number.
What are the Lyman, Balmer, and Paschen series, and how do they relate to atomic spectra?
-The Lyman, Balmer, and Paschen series are specific sets of spectral lines observed in the spectra of hydrogen. Each series corresponds to transitions of electrons between quantized energy levels and is named after the scientist who described them.
Outlines
🔬 Rutherford's Nucleus and Bohr's Quantum Leaps
This paragraph introduces the quantum model of the atom developed by Niels Bohr, which improved upon Rutherford's planetary model. It explains that electrons don't orbit the nucleus like planets but instead exist in quantized energy levels. Electrons can only occupy these specific levels and move between them by absorbing or emitting photons, which helps explain the observed spectral lines. The Bohr model is particularly effective for hydrogen and provides a foundation for understanding atomic structure.
🌟 The Balmer Series and Spectral Analysis
The second paragraph delves into the Balmer series, which is a part of the hydrogen spectrum. It discusses how the Bohr model predicts the energy levels at which electrons exist and how they transition between these levels by absorbing or emitting light of specific frequencies, which corresponds to the discrete spectral lines observed. The Balmer series is used as an example to illustrate how the model's predictions align with experimental observations, reinforcing the validity of the quantum model for hydrogen atoms.
Mindmap
Keywords
💡Atom
💡Electron
💡Nucleus
💡Energy levels
💡Quantum
💡Electromagnetic radiation
💡Spectrum
💡Periodic table
💡Protons
💡Neutrons
💡Spectroscopy
💡Bohr model
Highlights
Ernest Rutherford's gold foil experiment led to the discovery of the atom's positive, small nucleus.
Rutherford's model suggested electrons orbit the nucleus like planets around the sun.
Niels Bohr identified a flaw in Rutherford's model regarding electron movement and electromagnetic radiation.
Bohr's model introduced quantized energy levels for electrons, contradicting the smooth spectrum theory.
Electrons were observed to emit or absorb photons when transitioning between energy levels.
Bohr's model explained the discrete spectral lines observed in atomic spectra.
The atomic number indicates the number of protons in an atom.
The periodic table is based on the electron configuration, particularly the outermost electrons.
Bohr's model helps explain electron behavior in discrete energy states.
The number of neutrons in an atom can be estimated by subtracting the atomic number from the mass number.
Electrons in a neutral atom balance the number of protons.
Bohr's model suggests electrons jump between orbits, not orbit continuously.
Energy is required to move an electron to a higher energy level.
The Bohr model is a visual representation of quantized electron orbits.
Spectroscopic observations led to the discovery of spectral lines, such as the Lyman, Paschen, and Balmer series.
The Balmer series specifically explained discrete light units in the visible spectrum.
Bohr's model successfully predicted and matched the energy levels observed in spectral lines.
While Bohr's model is a foundational step, it only accurately represents hydrogen atoms.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: