The Quantum Mechanical model of an atom. What do atoms look like? Why?
TLDRThis script explores the evolution of atomic models, from Rutherford's planetary model to quantum mechanics. It explains how Bohr integrated Planck's quantization theory to propose stable electron orbits, and how de Broglie and Schrödinger further developed the understanding of electrons as waves, leading to the probability cloud model. The script delves into why electrons don't collapse into the nucleus and why atoms can't be squeezed together, highlighting the Heisenberg Uncertainty Principle and the reality of quantum mechanics as the foundation of our understanding of the atomic structure.
Takeaways
- 🌌 Great scientists like Newton and Rutherford have observed patterns in nature that led to significant scientific models, even if some were later refined or disproven.
- 🔬 The Rutherford model of the atom, which likened the atom to a mini solar system, was intuitive but ultimately incorrect due to the limitations of classical physics.
- 🌈 The classical model failed because it couldn't explain why electrons in orbit wouldn't radiate energy and collapse into the nucleus, as predicted by Maxwell's equations.
- 🔄 Max Planck's quantum theory introduced the concept of energy quantization, which was pivotal in addressing the issues with the classical atomic model.
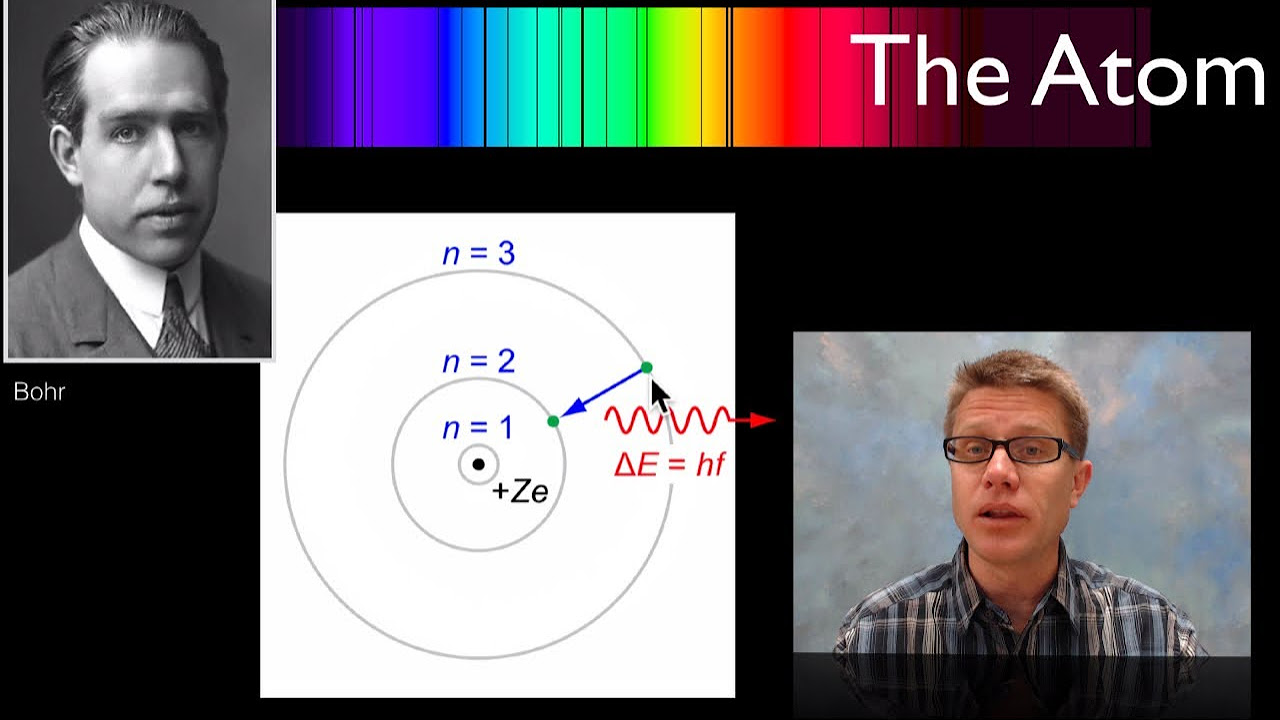
- 🌀 Neils Bohr proposed that electrons could exist in certain orbits without radiating energy, based on quantized angular momentum, which was a significant step towards a quantum model of the atom.
- 🌟 Louis de Broglie suggested that electrons behave both as particles and waves, leading to the idea that electrons exist in standing wave patterns around the nucleus.
- 📘 Erwin Schrodinger developed the wave equation that described the behavior of electrons as wave functions, which was more successful and comprehensive than the Bohr model.
- 🔬 Direct observation of atoms is impossible with visible light due to their small size, and indirect methods must be used to infer their structure.
- 🚫 Heisenberg's Uncertainty Principle prevents the exact determination of both the position and momentum of an electron simultaneously, meaning electrons form a probabilistic 'cloud' rather than a fixed orbit.
- 🌐 The electron cloud in an atom, as described by the Schrödinger equation, represents the probable location of an electron, with the highest probability at the Bohr radius.
- 💥 The inability to squeeze atoms together is due to the need to increase the electron's energy to a higher state, which requires an immense amount of energy.
- 🤔 Quantum mechanics, despite its unintuitive nature, provides a deeper understanding of reality and is fundamental to the behavior of the natural world.
Q & A
What pattern did Isaac Newton notice that was similar between a fired cannonball and the moon's orbit around the Earth?
-Isaac Newton realized that the forces acting on a fired cannonball are the same as those acting on the moon as it orbits the Earth, highlighting the universality of gravitational forces.
What was Ernest Rutherford's hypothesis regarding the structure of atoms?
-Ernest Rutherford hypothesized that atoms have a heavy nucleus, and he initially thought that electrons orbit the nucleus in a manner similar to how the moon orbits the Earth.
Why was the Rutherford model of the atom eventually deemed incorrect?
-The Rutherford model was incorrect because it suggested that electrons would radiate energy when orbiting the nucleus, which would cause them to spiral into the nucleus and collapse, contradicting the stable nature of atoms.
What was the significance of Max Planck's discovery in 1900 that led to a new understanding of atomic structure?
-Max Planck discovered that energy of photons is quantized, meaning that matter emits only discrete amounts of radiation. This concept was crucial for the development of quantum mechanics and the understanding of atomic structure.
How did Niels Bohr's model of the atom differ from Rutherford's model?
-Niels Bohr proposed that electrons could exist in certain special orbits without radiating energy, based on the quantization of angular momentum, which was a significant departure from the planetary model proposed by Rutherford.
What is the significance of Louis de Broglie's hypothesis about the nature of electrons?
-Louis de Broglie suggested that electrons are not just particles but also exhibit wave-like properties, which was a major philosophical leap and contributed to the development of the wave-particle duality concept in quantum mechanics.
What is the Schrödinger equation and why is it important in quantum mechanics?
-The Schrödinger equation is a fundamental equation in quantum mechanics that describes how quantum systems evolve over time. It is crucial because it allows for the calculation of the wave function of a system, which in turn determines the probabilities of different outcomes.
Why can't we directly observe the structure of atoms using visible light?
-Atoms are much smaller than the wavelength of visible light, so light passes through atoms without being reflected, making them invisible to the naked eye or conventional optical microscopes.
What does the Heisenberg Uncertainty Principle state and how does it relate to the behavior of electrons in atoms?
-The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know both the exact position and momentum of a particle. This principle explains why electrons form a probability cloud around the nucleus instead of being in a fixed orbit.
How do the images of hydrogen atoms taken by researchers in 2013 support the quantum mechanical model of the atom?
-The images, which are composites based on the trajectory of electrons emitted by hydrogen atoms, provide visual evidence that supports the quantum mechanical model where electrons form a cloud around the nucleus rather than existing in fixed orbits.
Why doesn't an electron fall into the nucleus despite the attractive electrostatic force between them?
-An electron does not fall into the nucleus because doing so would violate the Heisenberg Uncertainty Principle, which requires a balance between the precision of position and momentum measurements.
Why can't we squeeze atoms together to a point where they occupy no volume?
-Squeezing atoms to a smaller size would require increasing the energy of the electron to a higher energy state, which is impractical due to the immense amount of energy required, as demonstrated by the energy versus distance chart for an electron in an atom.
Outlines
🔬 The Evolution of Atomic Models
This paragraph introduces the historical development of atomic models, starting with Isaac Newton's recognition of universal forces and Ernest Rutherford's initial hypothesis of a solar system-like atom with a heavy nucleus. It explains the limitations of the Rutherford model, which suggested electrons orbiting the nucleus like planets, due to its contradiction with Maxwell's electromagnetic wave theory. The paragraph also highlights the introduction of quantum mechanics and Planck's quantization of energy, leading to Neils Bohr's hypothesis of quantized orbits for electrons without energy loss, marking a significant shift in understanding atomic structure.
🌌 Quantum Mechanics and the Bohr Model
The second paragraph delves into the quantum mechanics revolution, starting with Max Planck's discovery of energy quantization and moving on to Neils Bohr's model, which proposed special orbits for electrons based on quantized angular momentum. It discusses the limitations of the Bohr model, such as its inability to explain why these orbits are stable or why electrons do not emit photons continuously. The paragraph also introduces Louis de Broglie's hypothesis that electrons exhibit wave-like properties, setting the stage for a new understanding of atomic structure through wave-particle duality.
🌐 The Wave-Particle Nature of Electrons and the Schrödinger Equation
This paragraph explores the concept of wave-particle duality further, with de Broglie's suggestion that electrons can only exist in orbits where their wave properties allow for constructive interference. It then introduces Erwin Schrödinger's groundbreaking wave equation, which provides a more accurate and comprehensive description of atomic structure than the Bohr model. The paragraph explains how the Schrödinger equation supports the idea of the electron existing as a probability cloud around the nucleus, challenging the classical view of discrete particles in fixed orbits.
📸 Imaging Atoms and the Implications of Quantum Mechanics
The final paragraph discusses the challenges of directly imaging atoms due to their small size and the limitations imposed by the Heisenberg Uncertainty Principle. It describes how researchers used a composite image based on electron trajectories to indirectly visualize hydrogen atoms, providing evidence for the quantum mechanical model. The paragraph also addresses common questions about atomic structure, such as why electrons don't fall into the nucleus and why atoms can't be squeezed together, explaining these phenomena through the principles of quantum mechanics. It concludes with a reflection on the unintuitive yet fundamental nature of quantum mechanics in understanding the fabric of reality.
Mindmap
Keywords
💡Quantum Mechanics
💡Rutherford Model
💡Electron
💡Nucleus
💡Bohr Model
💡Wave-Particle Duality
💡Schrodinger Equation
💡Heisenberg Uncertainty Principle
💡Angular Momentum
💡Energy Levels
💡Quantum States
Highlights
Isaac Newton's realization that the forces acting on a fired cannonball are the same as those on the moon orbiting the Earth.
Ernest Rutherford's hypothesis that the moon's orbit around the Earth is similar to an electron's orbit around the nucleus of an atom.
The Rutherford model's depiction of the atom, despite being outdated, remains the most familiar image of an atom.
The true appearance of an atom is vastly different from the Rutherford model, challenging the notion of mostly empty space.
Quantum mechanics is introduced as the key to understanding the true nature of atoms.
Ernest Rutherford's planetary model of the atom, which likened the atom to a mini solar system.
The problem with the Rutherford model due to the predicted emission of electromagnetic waves by orbiting electrons.
Max Planck's quantization of energy, which laid the groundwork for quantum mechanics.
Neils Bohr's hypothesis allowing electrons to exist in special orbits without radiating energy, based on Planck's constant.
Bohr's model's prediction of electron orbits and energy emissions, which were confirmed by observations.
Louis de Broglie's proposal that electrons are waves, leading to the concept of matter waves.
Erwin Schrodinger's development of the wave equation, which revolutionized the understanding of quantum mechanics.
The inability to directly observe atoms due to their small size relative to visible light wavelengths.
Heisenberg's Uncertainty Principle and its implications for the behavior of electrons within atoms.
The electron cloud model, which describes the probable position of electrons around the nucleus.
The size comparison between the electron cloud and the proton cloud, emphasizing the vast difference due to mass.
The impossibility of directly imaging a hydrogen atom without altering its state.
The indirect imaging of hydrogen atoms using electron trajectories, providing evidence for the 3D model.
The explanation of why electrons do not fall into the nucleus despite the attractive force.
The energy required to compress atoms and the reason why solid objects maintain their form.
Quantum mechanics as the fundamental theory explaining the deeper reality of the universe.
The promotion of the 'Quantum Objects' class on Brilliant for a deeper understanding of quantum physics.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: