Wave Model of an Electron
TLDRIn this AP Physics essentials video, Mr. Andersen explores the wave model of an electron, which helps explain electron behavior as waves rather than particles. The concept of standing waves is introduced, where electrons' motion creates interference patterns, allowing them to exist only in specific energy states. The de Broglie wavelength is key to understanding electron momentum and the atom's size. The video also connects the wave model to the emission of photons when electrons transition between energy levels, which is observed as spectral lines. This model offers a qualitative link between electron waves and atomic energy states, providing a deeper insight into quantum mechanics.
Takeaways
- 🌌 Electrons exhibit wave-like behavior rather than particle-like, which is crucial for understanding quantum phenomena.
- 🌐 The wave model of an electron helps explain the discrete energy levels and the absence of electrons between these levels in a Bohr atom model.
- 🎶 Electrons can create standing waves due to their rapid motion, which interferes with themselves, forming stable energy states around the atom.
- 🔢 The de Broglie wavelength is a fundamental concept that describes the wave nature of electrons and is used to calculate their momentum.
- 🌈 Only integer multiples of the de Broglie wavelength allow for the stable existence of electrons in orbits; otherwise, they annihilate themselves.
- 💡 The energy difference between electron energy levels is released as photons when electrons transition to lower levels, observable as spectral lines.
- 🎸 The concept of fundamentals and harmonics in wave physics, like strumming a guitar, can be used as an analogy for understanding electron energy states.
- 📏 The circumference of the electron's orbit can be calculated using the de Broglie wavelength, which aligns with the Bohr radius.
- 🔄 Electrons can only exist in certain energy states due to the requirement of whole integer wavelengths fitting into their orbital paths.
- 🚀 The absorption of a photon is necessary for an electron to jump to a higher energy level, which is part of the explanation for observed spectral lines.
- 🔑 The wave model qualitatively links the behavior of electrons to the different energy states within an atom, providing a deeper understanding of quantum mechanics.
Q & A
How do electrons behave at the quantum level according to the script?
-At the quantum level, electrons do not behave like particles but act more like waves, which is explained by the wave model of an electron.
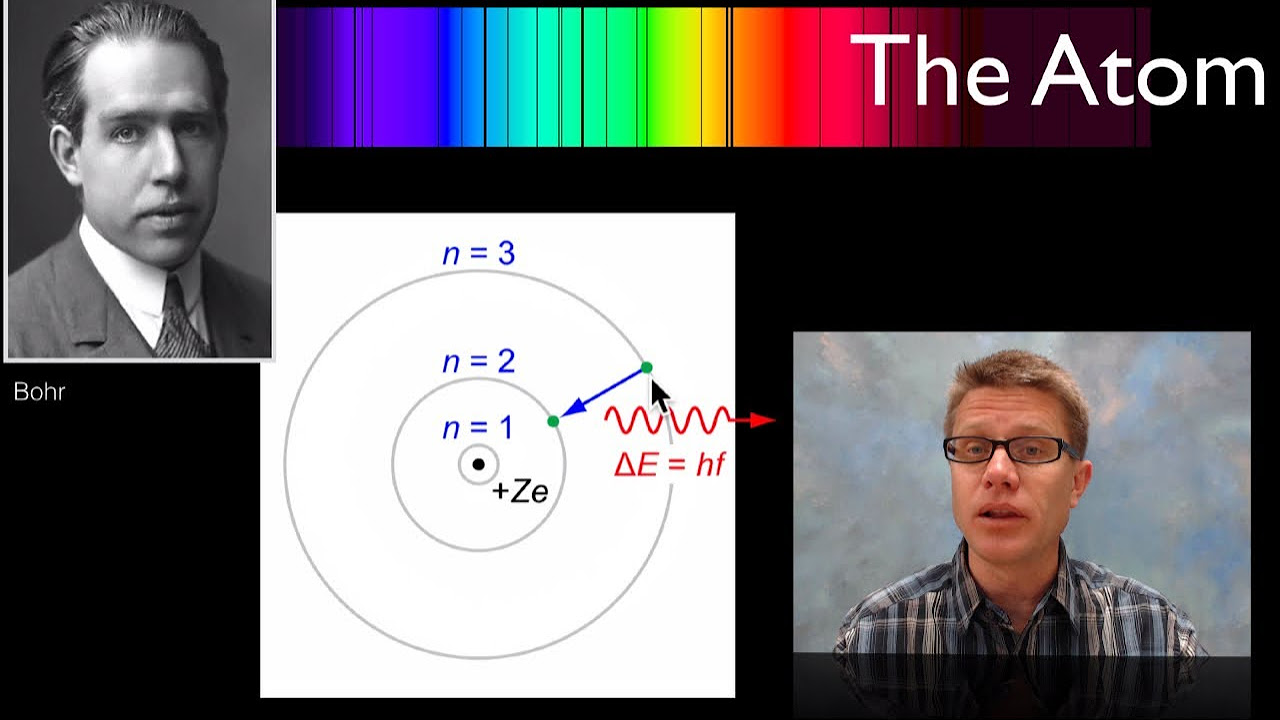
What was puzzling scientists about the behavior of electrons in a Bohr atom?
-Scientists were puzzled by the fact that electrons could only exist at certain discrete locations (here or here) and not in between, which was later explained by the wave model.
What is a standing wave and how does it relate to the behavior of electrons around an atom?
-A standing wave is a wave pattern that occurs when waves interfere with themselves. It is hypothesized that electrons moving around an atom could be interfering with themselves, creating standing waves, which is how they can exist at certain energy levels.
Why do electrons annihilate themselves if the de Broglie wavelength does not match up perfectly between orbits?
-If the de Broglie wavelength does not match up perfectly between orbits, the waves do not constructively interfere, leading to the electron's wave function collapsing, or 'annihilating' itself.
How does the wave model help explain the emission of a photon when an electron falls to a lower energy level?
-The wave model helps explain this phenomenon by suggesting that the energy contained within the standing wave is released when an electron transitions to a lower energy level, and this energy is emitted as a photon.
What is the significance of the de Broglie wavelength in understanding the behavior of electrons?
-The de Broglie wavelength is significant because it allows us to calculate the momentum of an electron and understand the conditions under which electrons can exist in certain energy states around an atom.
How does the script relate the concept of fundamentals and harmonics in music to the behavior of electrons?
-The script uses the analogy of musical fundamentals and harmonics to explain how electrons with different wavelengths can exist around an atom, with each 'harmonic' corresponding to a different energy level or orbit.
What is the Bohr radius, and how does it relate to the size of an atom as explained in the script?
-The Bohr radius is a measure of the size of an atom, specifically the distance from the nucleus to the electron in the lowest energy level. The script explains that by calculating the de Broglie wavelength and using it in the circumference equation, the size of the atom matches the Bohr radius of 0.53 angstroms.
How does the script explain the process of an electron falling to a lower energy level and releasing energy?
-The script explains that as electrons fall to a lower energy level, the energy that was built up in the standing wave at the higher level is released in the form of a photon, which corresponds to the spectral lines observed.
What is the role of photons in the process of electrons transitioning between energy levels, as described in the script?
-Photons play a crucial role as they are the carriers of energy during electron transitions. When an electron falls to a lower energy level, it releases a photon with the energy difference between the levels. Conversely, to jump back up to a higher level, an electron must absorb a photon.
How does the script help in understanding the spectral lines observed in atoms?
-The script helps in understanding spectral lines by explaining that they are the result of electrons transitioning between energy levels and releasing or absorbing photons. This process is linked to the standing wave model and the energy contained within it.
Outlines
🌌 Wave Model of an Electron
This paragraph introduces the wave model of an electron, emphasizing its wave-like behavior rather than particle-like. It explains how this model helps us understand the behavior of electrons in a Bohr atom and the concept of standing waves. The idea that electrons might interfere with themselves to create standing waves is introduced, which is key to understanding electron energy states. The paragraph also touches on the de Broglie wavelength, which is crucial for calculating momentum and the size of the atom. Additionally, it explains how electrons emit photons of light when they fall to lower energy levels, which is observed as spectral lines.
Mindmap
Keywords
💡Wave model
💡Electron
💡Bohr atom
💡Standing wave
💡de Broglie wavelength
💡Energy states
💡Photon
💡Spectral lines
💡Fundamentals and harmonics
💡Momentum
💡Quantum mechanics
Highlights
Electrons behave more like waves than particles at the quantum level.
The wave model helps to understand the behavior of electrons in a Bohr atom.
Electrons can only exist in certain places without being in the middle, which was puzzling to scientists.
The wave model explains why electrons emit a photon of light when they fall to a lower energy level.
Standing waves are created when waves interfere with themselves.
Electrons may create standing waves by moving fast and interfering with themselves.
Standing waves allow electrons to exist in certain energy states.
Electrons must have an integer number of de Broglie wavelengths to exist.
If the de Broglie wavelength does not match perfectly, the electron annihilates itself.
The de Broglie wavelength can be used to calculate the momentum and size of an atom.
Electrons falling to a lower energy level release energy in the form of a photon.
Spectral lines can be observed when energy from standing waves is released as photons.
Fundamentals and harmonics can be used as a model to understand electron behavior.
The first inner electron has one wavelength wrapping around the atom.
The de Broglie wavelength can be used to calculate the size of the atom, matching the Bohr radius.
Higher energy levels have more wavelengths, leading to different de Broglie wavelengths.
Electrons race around, interfering with themselves, building up energy in one energy level.
Electrons release energy as photons when they fall back down to a higher energy level.
Absorption of photons is necessary for electrons to jump back up to a higher energy level.
The video aims to qualitatively link the wave model for electrons with different energy states in an atom.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: