How to Speak Chemistrian: Crash Course Chemistry #11
TLDRThis video uses an analogy of waking up in Belgium and hearing different languages to explain chemistry terminology, which can seem like a foreign language. It provides an overview of how to name and write formulas for ions and acids. It covers topics like how elements gain or lose electrons to become cations or anions, how transition metals use Roman numerals in naming, and how prefixes like 'hypo' and 'per' denote the number of oxygen atoms in acid names. The periodic table serves as a guide, with metals on the left forming cations and nonmetals on the right forming anions. Learning the naming rules takes practice, but this video aims to provide a helpful starting point.
Takeaways
- 😃 Chemistry has its own language called 'Chemistrian' with unique rules for naming/writing chemical formulas.
- 🤓 Atoms become positively or negatively charged ions by losing/gaining electrons.
- 📝 Cations and anions are named using different conventions.
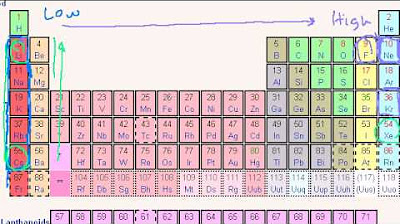
- 🌐 The Periodic Table acts as a 'phrasebook' for the language of chemistry.
- 😮 Transition metals use Roman numerals in their ion names.
- 😬 Acids always contain hydrogen cations.
- 👂 Anions and acids are named by their oxygen content using prefixes/suffixes.
- 📖 There's a handy table to remember naming rules for acids/anions.
- 🧠 Applying naming rules is like waking up in a Belgian meadow speaking an unfamiliar language!
- 🙌 Now you know key rules for writing/naming ions, transition metals and acids in chemistry.
Q & A
What is chemistrian?
-Chemistrian is the term used in the video to refer to the unique language and naming system used in chemistry. It consists of symbols, formulas, prefixes, suffixes, and rules for translating chemical names into spoken words.
How are monatomic ions named?
-Monatomic ions are named using the root chemical symbol for the atom, followed by its ion charge as a superscript. Cations just add the word 'ion' while anions end in '-ide'.
Why are Roman numerals used for transition metal ions?
-Roman numerals are used when naming transition metal ions to indicate the ion's charge, since Arabic numerals are already used extensively in chemistry for other purposes.
What is the naming convention for oxyacids?
-Oxyacids are named using the root chemical name, followed by suffixes that indicate the number of oxygen atoms. '-ic' acids have the baseline oxygen amount, '-ous' means less oxygen, 'hypo-' means even fewer, and 'per-' means more oxygen.
How can you determine the chemical formula of an acid?
-The formula of an acid can be determined by identifying the anion, which will bond with H+ cations. The anion's name also indicates how many oxygen atoms are present.
Where are anion-forming elements found on the periodic table?
-Anion-forming elements like oxygen, sulfur, and the halogens are found on the right side of the periodic table.
What charge will alkali metal ions typically have?
-+1, as alkali metals tend to lose a single electron when forming cations.
What is the difference between '-ate' and '-ite' suffixes?
-'-ate' indicates the baseline oxygen amount, while '-ite' indicates less oxygen than the '-ate' form.
What does the 'hypo-' prefix signify?
-'Hypo-' indicates two fewer oxygen atoms than the '-ic' acid form.
What is an easy way to remember naming patterns?
-Using the table provided, which summarizes the key suffixes, prefixes, and their meanings regarding oxygen content.
Outlines
😀 Imagining waking up in Belgium to illustrate learning chemistry
The paragraph begins by imagining waking up under a tree in Belgium, not understanding the languages being spoken. This experience of confusion is compared to learning chemistry, which has its own unique language system to describe chemicals. Just as there are dialects in different parts of Belgium, there are rules in chemistry that change depending on location in the periodic table.
😃 Using roman numerals and prefixes/suffixes to name transition metal ions and acids
The paragraph explains the use of roman numerals to denote the charge of transition metal ions, with examples like Iron II and Iron III. It then discusses naming acids and anions using prefixes like "hypo" and suffixes like "-ate", which indicate the number of oxygen atoms. A table summarizes these naming rules.
😊 Reviewing key learnings about chemical formulas, names, and concepts
The paragraph summarizes the main topics covered in the video, including determining formulas and names for monatomic ions, locating cation/anion-forming elements on the periodic table, naming transition metals and acids/anions, and using prefixes and suffixes to indicate oxygen atoms.
Mindmap
Keywords
💡chemistrian
💡ionic compounds
💡cations
💡anions
💡monatomic ions
💡transition metals
💡acids
💡anions
💡oxygen content
💡chemical formulas
Highlights
First significant research finding
Introduction of new theoretical model
Description of innovative experimental method
Key conclusions and practical applications
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: