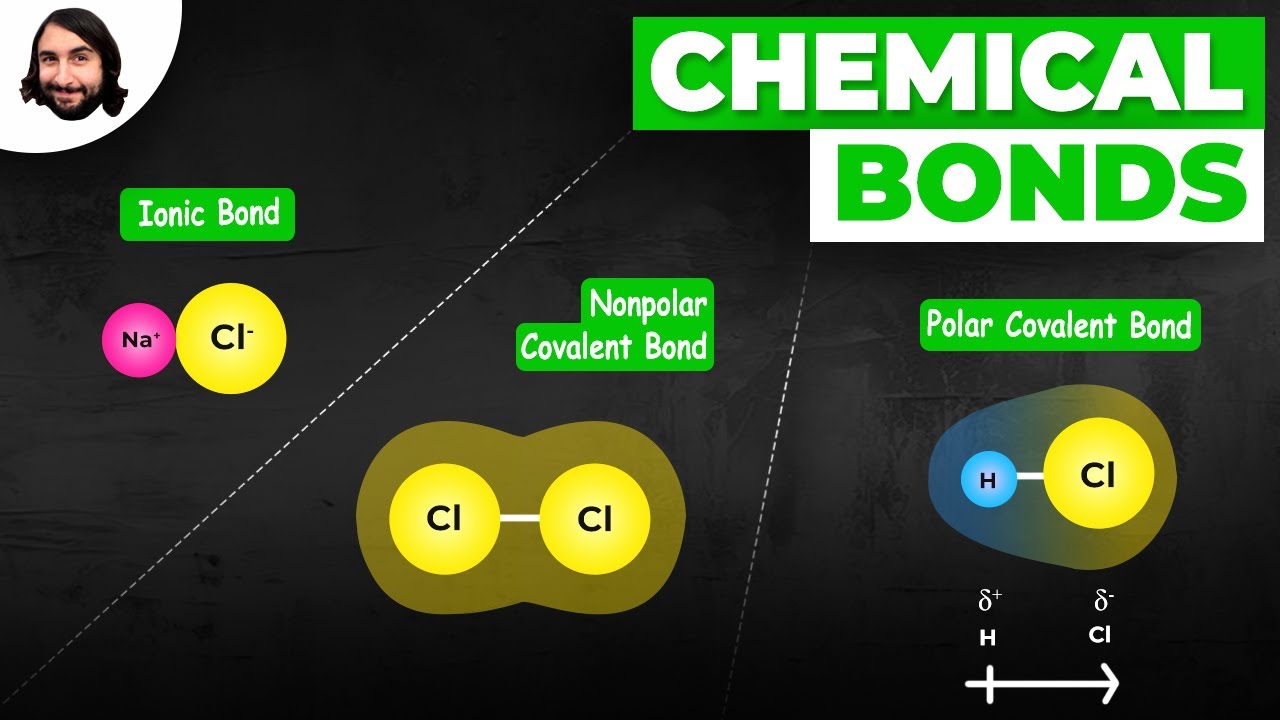
Polar and Nonpolar Covalent Bonds
TLDRThe video script delves into the concept of polar and nonpolar covalent bonds, which are formed between non-metal elements. It explains that electronegativity, the measure of an element's ability to attract electrons, plays a crucial role in determining the type of bond. When two atoms with the same electronegativity share electrons equally, a non-polar bond is formed, as seen in a hydrogen-hydrogen bond. Conversely, a polar bond occurs when there's an uneven distribution of electrons due to a difference in electronegativity, resulting in partial charges, exemplified by a hydrogen-chlorine bond. The video uses the periodic table and electronegativity values to illustrate these concepts, highlighting that polar bonds have electrons closer to the more electronegative atom, creating a partial negative charge on that atom and a partial positive charge on the other. The key takeaway is understanding the negative charge of electrons and how electronegativity influences electron sharing, leading to either polar or non-polar covalent bonds.
Takeaways
- 🔬 Covalent bonds form between non-metal elements, where electrons are shared between atoms.
- 🌈 Non-metals in the periodic table are color-coded based on their electronegativity, which indicates their tendency to attract electrons.
- ⚔️ Electronegativity is depicted as a 'sword', symbolizing how strongly an element wants to 'steal' electrons.
- 🔝 The higher the electronegativity, the more an element attracts electrons, with fluorine having the highest electronegativity.
- ⚖️ In covalent bonds, electrons are not always shared evenly due to differences in electronegativity between the atoms.
- ⚡ When electrons are closer to an atom with higher electronegativity, that atom gets a partial negative charge, and the other a partial positive charge.
- 💠 A non-polar covalent bond occurs when atoms have the same or very similar electronegativity, resulting in an even sharing of electrons with no partial charges.
- ⚡️ A polar covalent bond occurs when there is a significant difference in electronegativity, leading to an uneven sharing of electrons and partial charges.
- 🌟 Examples given include H-H (non-polar), H-Cl (polar), Cl-Cl (non-polar), H-O (polar), O-O (non-polar), C-O (polar), and C-H (non-polar).
- 📊 The degree of the charges in polar bonds isn't specified, only that they exist, and range between zero and one.
- 🔍 To determine if a bond is polar or non-polar, consider the electronegativity values and whether they are shared equally or attracted more to one atom.
Q & A
What are polar and nonpolar covalent bonds?
-Polar and nonpolar covalent bonds are specific types of covalent bonds that involve the sharing of electrons between non-metal elements. In a polar covalent bond, the electrons are not shared evenly due to a difference in electronegativity between the atoms, leading to partial charges. In a nonpolar covalent bond, the electrons are shared evenly because the atoms have the same or similar electronegativity, resulting in no partial charges.
What elements typically form covalent bonds?
-Non-metal elements typically form covalent bonds. These elements are found on the right side of the periodic table and include elements such as hydrogen, oxygen, nitrogen, fluorine, and chlorine.
What is electronegativity and how does it relate to covalent bonding?
-Electronegativity is a measure of how strongly an atom attracts electrons in a chemical bond. It is indicated by a numerical value, with higher values indicating a stronger attraction for electrons. In covalent bonding, the difference in electronegativity between the bonding atoms determines whether the bond is polar or nonpolar. A greater electronegativity difference leads to a polar bond, while equal or similar electronegativities result in a nonpolar bond.
How can you identify a polar covalent bond?
-A polar covalent bond can be identified by the unequal sharing of electrons between the atoms. This occurs when there is a significant difference in electronegativity between the bonding atoms, causing the electrons to be pulled closer to the atom with the higher electronegativity. As a result, this atom will have a partial negative charge, and the other atom will have a partial positive charge.
What is the difference in electron sharing between polar and nonpolar covalent bonds?
-In a polar covalent bond, the electrons are not shared evenly due to the difference in electronegativity between the atoms, leading to partial charges. In contrast, in a nonpolar covalent bond, the electrons are shared evenly because the atoms have similar electronegativities, resulting in no partial charges.
How does the electronegativity of elements affect the polarity of a covalent bond?
-The electronegativity of elements directly affects the polarity of a covalent bond. If one atom has a significantly higher electronegativity than the other, it will attract the shared electrons more strongly, causing the electrons to spend more time near that atom. This unequal distribution of electron density leads to a polar bond with partial charges. If the electronegativities are similar, the electrons are shared more evenly, resulting in a nonpolar bond with no partial charges.
What happens when two non-metals with the same electronegativity bond together?
-When two non-metals with the same electronegativity bond together, the electrons are shared evenly between the atoms. Since there is no difference in the attraction for electrons, the electrons are not pulled towards one atom more than the other, resulting in a nonpolar covalent bond with no partial charges.
Can you give an example of a nonpolar covalent bond?
-An example of a nonpolar covalent bond is a bond between two hydrogen atoms (H-H). Since both hydrogen atoms have the same electronegativity, the electrons are shared evenly, and there are no partial charges, making it a nonpolar bond.
What is the role of electronegativity in determining partial charges in covalent bonds?
-Electronegativity plays a crucial role in determining partial charges in covalent bonds. The difference in electronegativity between the bonding atoms dictates whether the shared electrons will be unevenly distributed, leading to partial charges. If one atom has a higher electronegativity, it will attract the electrons more, resulting in a partial negative charge on that atom and a partial positive charge on the other atom, creating a polar bond.
How can you predict the polarity of a bond between two given non-metal elements?
-To predict the polarity of a bond between two non-metal elements, you can compare their electronegativities. If the electronegativities are equal or very similar, the bond will be nonpolar because the electrons will be shared evenly. If there is a significant difference in electronegativity, the bond will be polar, with the electron density skewed towards the atom with the higher electronegativity, resulting in partial charges.
What is the significance of understanding polar and nonpolar covalent bonds in chemistry?
-Understanding polar and nonpolar covalent bonds is fundamental in chemistry as it helps in predicting the behavior of molecules, their reactivity, and physical properties such as solubility and boiling points. Polar molecules often have different solubility properties in water compared to nonpolar molecules, and this understanding is crucial in various chemical and industrial processes, as well as in the study of molecular structures and interactions.
Outlines
🔬 Understanding Polar and Nonpolar Covalent Bonds
This paragraph introduces the concept of polar and nonpolar covalent bonds, which are specific types of covalent bonds formed between non-metal elements. The speaker explains that even though electrons are shared in covalent bonds, the sharing is not always even due to differences in electronegativity. Electronegativity is described as the ability of an element to attract electrons, with fluorine having the highest electronegativity. The paragraph provides examples to illustrate how the electrons are shared more by the more electronegative atom, resulting in partial charges and thus polar bonds, whereas equal sharing results in nonpolar bonds.
🌟 Examples of Polar and Nonpolar Bonds
The second paragraph delves into examples to further clarify the concept of polar and nonpolar bonds. It discusses how when two atoms of the same non-metal element bond, such as chlorine or oxygen, the electrons are shared equally, resulting in nonpolar bonds. In contrast, when different non-metals bond, such as hydrogen and oxygen, the greater electronegativity of oxygen pulls the shared electrons closer, creating a polar bond with partial charges. The paragraph emphasizes the importance of understanding electronegativity and how it influences the sharing of electrons to determine if a bond is polar or nonpolar.
📚 Review of Polar and Nonpolar Covalent Bonds
The final paragraph reviews the differences between polar and nonpolar covalent bonds. It reiterates that in polar covalent bonds, electrons are not shared evenly due to the higher electronegativity of one atom, leading to partial charges. Conversely, nonpolar covalent bonds occur between atoms with similar electronegativity, resulting in equal sharing of electrons and no partial charges. The paragraph reinforces the key takeaways: recognizing the negative charge of electrons, understanding electronegativity, and its impact on electron sharing to determine bond polarity.
Mindmap
Keywords
💡Covalent Bonds
💡Electronegativity
💡Nonpolar Covalent Bonds
💡Polar Covalent Bonds
💡Partial Charges
💡Periodic Table
💡Non-Metals
💡Sharing of Electrons
💡Charge of Electrons
💡Hydrogen Bonding
💡Carbon-Oxygen Bond
Highlights
Polar and nonpolar covalent bonds are specific types of covalent bonds formed between non-metal elements.
Electronegativity determines how an element attracts electrons in a covalent bond.
Higher electronegativity results in electrons being pulled closer to the more electronegative atom.
When electrons are shared evenly, the bond is nonpolar, as seen with hydrogen bonded to hydrogen.
Polar bonds occur when electrons are not shared evenly, leading to partial charges on the atoms.
Chlorine has a higher electronegativity than hydrogen, making their bond polar.
Two atoms of the same non-metal with equal electronegativity form a nonpolar bond.
Oxygen's higher electronegativity compared to hydrogen results in a polar bond.
Bonds between atoms of the same element are always nonpolar due to equal electronegativity.
The difference between polar and nonpolar bonds lies in the evenness of electron sharing and the presence of partial charges.
In a polar bond, the more electronegative atom has a partial negative charge, and the other has a partial positive charge.
Carbon and oxygen bond is an example of a polar bond due to the difference in their electronegativities.
Carbon and hydrogen bond is considered nonpolar due to their very similar electronegativities.
The charge of electrons is negative, influencing the polarity of covalent bonds.
Electronegativity differences determine how electrons are shared between atoms in a covalent bond.
Understanding electronegativity is key to predicting the polarity of covalent bonds.
The video concludes with a review of the similarities and differences between polar and nonpolar covalent bonds.
Transcripts
Browse More Related Video

Introduction to Ionic Bonding and Covalent Bonding

Types of Chemical Bonds - AP Chem Unit 2, Topic 1
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar

Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy

Which Bond Is More Polar?
5.0 / 5 (0 votes)
Thanks for rating: