Types of Chemical Bonds - AP Chem Unit 2, Topic 1
TLDRThis video script delves into the world of chemical bonding, focusing on the types of bonds formed between elements. It explains covalent bonds, which occur when two non-metals share electrons, leading to a stable octet structure. The script illustrates how the sharing of electrons can be equal in nonpolar covalent bonds, such as between iodine and sulfur, or unequal in polar covalent bonds, exemplified by the bond between carbon and fluorine. The difference in electronegativity between atoms determines whether a bond is polar or nonpolar, with a difference of less than 0.5 indicating nonpolarity. Ionic bonds, where a metal transfers electrons to a non-metal, are also discussed. Finally, the script touches on metallic bonding, characterized by the free movement of electrons among metal atoms, which accounts for metals' excellent electrical conductivity. The lesson concludes with an invitation to continue learning in the next video.
Takeaways
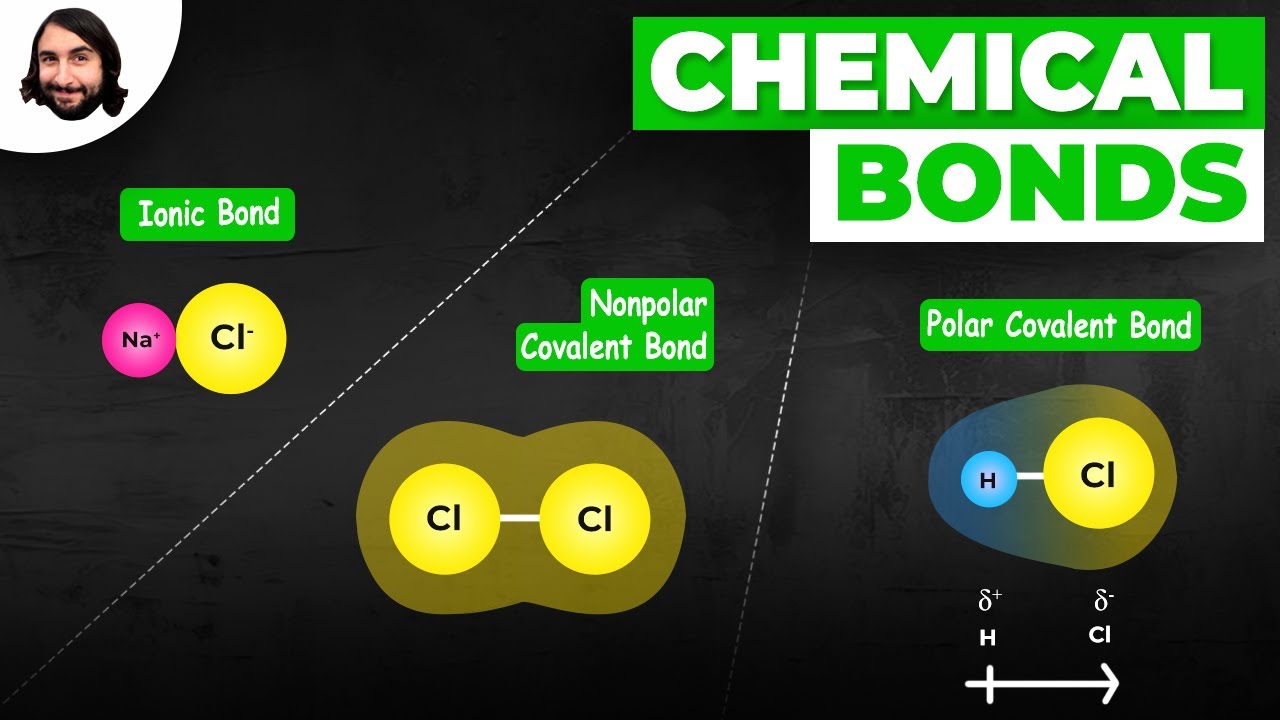
- 🔬 Covalent bonds occur when two non-metal atoms share a pair of electrons, aiming for a stable octet structure.
- 🔋 When atoms are far apart and not bonding, they have a higher potential energy compared to when they are closer and bonded.
- 📈 The formation of a chemical bond typically results in a lower potential energy state, which is more stable.
- 🏷 A single line in a structural formula represents a covalent bond, with double or triple lines indicating double or triple bonds, respectively.
- ⚖️ Nonpolar covalent bonds involve equal or nearly equal sharing of electrons between two non-metal atoms.
- 🔌 Polar covalent bonds occur when there is an unequal sharing of electrons due to a difference in electronegativity, with one atom 'hogging' the electrons.
- 📊 The electronegativity difference between two atoms determines if a bond is polar or nonpolar; differences less than 0.5 are considered nonpolar.
- 🚫 When there's a metal and a non-metal involved, the bond is ionic, with the metal transferring electrons to the non-metal.
- 🤝 Metallic bonding involves delocalized electrons that are free to move around the metal lattice, contributing to its conductivity.
- 🧊 Metallic elements have a fixed nucleus position but allow electrons to move freely, which explains their malleability and ductility.
- ⚡ Good electrical conductivity in metals is due to the free movement of delocalized electrons, which is essential for the flow of electric current.
Q & A
What is a covalent bond?
-A covalent bond is a type of chemical bond formed when two non-metal atoms share a pair of electrons, allowing each atom to achieve a stable electron configuration, typically an octet.
How is the potential energy of atoms related to the formation of a covalent bond?
-When two atoms are far apart and not bonding, they have a higher potential energy. As they come closer together and form a bond, they reach a lower potential energy state. The lowest potential energy state is typically when the atoms are bonded.
What does a single line between two atoms in a chemical structure represent?
-A single line between two atoms in a chemical structure represents a covalent bond, indicating that the atoms are sharing a pair of electrons.
What is the difference between a nonpolar and a polar covalent bond?
-A nonpolar covalent bond occurs when two non-metals share electrons equally or almost equally. A polar covalent bond occurs when the electrons are shared unequally, with one atom 'hogging' the electrons more than the other.
How is electronegativity used to determine if a bond is polar or nonpolar?
-The difference in electronegativity between the two atoms in a bond is used to determine if it is polar or nonpolar. If the difference is less than about 0.5, the bond is considered nonpolar. If the difference is 0.5 or greater, the bond is considered polar.
What is an ionic bond?
-An ionic bond is a type of chemical bond that typically occurs between a metal and a non-metal, where the metal transfers one or more electrons to the non-metal, resulting in the formation of positively and negatively charged ions that are attracted to each other.
What is metallic bonding?
-Metallic bonding is a type of chemical bonding that occurs in metals. It involves the delocalization of valence electrons, which are free to move throughout the metal lattice, allowing metals to be good conductors of electricity and heat.
Why are metals good conductors of electricity?
-Metals are good conductors of electricity because the valence electrons in a metallic bond are delocalized and can move freely throughout the metal lattice, facilitating the flow of electric charge.
What is the significance of the octet rule in the context of covalent bonding?
-The octet rule states that atoms are most stable when they have eight electrons in their valence shell. Covalent bonding often involves the sharing of electrons between atoms to achieve this stable electron configuration.
How does the electronegativity difference between carbon and hydrogen affect their covalent bond?
-The electronegativity difference between carbon (2.5) and hydrogen (2.1) is 0.4, which is less than 0.5. This suggests a nonpolar covalent bond, although carbon may slightly 'hog' the electrons, resulting in a sharing arrangement that is close to but not exactly 50-50.
What is the role of electron delocalization in metallic bonding?
-Electron delocalization in metallic bonding refers to the ability of valence electrons to move freely among the metal atoms. This freedom of movement is what allows metals to be malleable, ductile, and good conductors of electricity and heat.
Outlines
🔬 Understanding Covalent Bonds
This paragraph introduces covalent bonds, which occur when two non-metal atoms share a pair of electrons to achieve a stable octet. The example of iodine and chlorine is used to illustrate how these atoms share electrons equally, resulting in a nonpolar covalent bond. The concept of potential energy is discussed, with a higher potential energy state when atoms are far apart and a lower one when they are close and bonded. The representation of covalent bonds through lines in chemical structures is explained, with single, double, or triple lines indicating the number of shared electron pairs. The difference between polar and nonpolar covalent bonds is also introduced, with electronegativity being the key factor in determining the type of bond.
🔋 Electronegativity and Bond Polarity
The second paragraph delves into electronegativity, explaining its role in determining whether a bond is polar or nonpolar. It is clarified that a difference in electronegativity of less than 0.5 typically results in a nonpolar covalent bond, while a difference of 0.5 or greater indicates a polar covalent bond. The more electronegative atom is described as 'hogging' the electrons, leading to a partial negative charge on that side of the bond. The concept of ionic bonds is introduced when a metal and a non-metal are involved, with metals tending to transfer electrons to non-metals. Examples using boron and chlorine, carbon and hydrogen, and sodium and bromine are provided to illustrate these principles. Additionally, metallic bonding is explained, highlighting the free movement of electrons among metal atoms, which contributes to metals' ability to conduct electricity.
Mindmap
Keywords
💡Covalent bond
💡Non-metals
💡Valence electrons
💡Potential energy
💡Nonpolar covalent bond
💡Polar covalent bond
💡Electronegativity
💡Ionic bond
💡Metallic bonding
💡Delocalized electrons
💡Electron sharing
Highlights
Covalent bonds are formed when two non-metals share electrons.
Atoms with eight valence electrons achieve a stable octet configuration.
Potential energy is higher when atoms are far apart and decreases upon bonding.
A line in chemical structure diagrams represents a shared electron pair in a covalent bond.
Double or triple bonds are indicated by two or three lines respectively.
Nonpolar covalent bonds occur when two nonmetals share electrons equally.
Polar covalent bonds result from unequal sharing of electrons between two nonmetals.
Electronegativity differences determine if a bond is polar or nonpolar.
A difference in electronegativity less than 0.5 typically indicates a nonpolar bond.
A difference in electronegativity of 0.5 or greater indicates a polar bond.
The atom with higher electronegativity in a polar bond has a partial negative charge.
Ionic bonds occur between a metal and a non-metal, with the metal transferring electrons.
Metallic bonding involves delocalized electrons that can move freely within a metal.
Metals and alloys are good conductors of electricity due to the free movement of electrons.
Metallic bonding is characterized by a sea of delocalized electrons around fixed metallic nuclei.
Electronegativity values help predict the type of chemical bond formed between atoms.
Boron and chlorine form a polar covalent bond due to a significant difference in electronegativity.
Carbon and hydrogen form a nonpolar covalent bond as their electronegativity difference is less than 0.5.
Transcripts
Browse More Related Video

Introduction to Ionic Bonding and Covalent Bonding

Polar and Nonpolar Covalent Bonds

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy

Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar

Ionic Bonds, Polar Covalent Bonds, and Nonpolar Covalent Bonds
5.0 / 5 (0 votes)
Thanks for rating: