Introduction to Ionic Bonding and Covalent Bonding
TLDRThe video script offers an insightful exploration into the fundamental differences between ionic and covalent bonding. Ionic bonds are formed through the transfer of electrons from one atom to another, typically resulting in the formation of ions. This process is exemplified by the reaction between sodium and chlorine, where sodium donates an electron to chlorine, leading to the creation of a positively charged sodium ion and a negatively charged chloride ion. These ions are then attracted to each other due to the electrostatic force between opposite charges, forming an ionic bond. In contrast, covalent bonds involve the sharing of electrons between atoms, as seen in the bond between two hydrogen atoms, which is nonpolar due to the equal sharing of electrons. However, when there is an unequal sharing due to a difference in electronegativity, as in the bond between hydrogen and fluorine, a polar covalent bond is formed. The script also provides a practical approach to classifying bonds as ionic, polar covalent, or non-polar covalent by considering the elements involved and their position in the periodic table, as well as the electronegativity difference. This educational content is designed to enhance understanding of chemical bonding, making it an engaging and informative watch for viewers interested in chemistry.
Takeaways
- 🔬 Ionic bonding involves the transfer of electrons from one atom to another, resulting in the formation of ions.
- 📊 Sodium (Na) and Chlorine (Cl) are used as examples to illustrate ionic bonding, where Na donates an electron to Cl, forming Na⁺ and Cl⁻ ions.
- 🧲 Ionic bonds are formed due to the electrostatic attraction between oppositely charged ions, with like charges repelling and opposite charges attracting.
- 🤝 Covalent bonding involves the sharing of electrons between atoms, as seen in the bond between two hydrogen atoms.
- 📚 Hydrogen atoms have one valence electron and aim to fill their outer shell with two electrons, sharing this electron to form a covalent bond.
- ⚖️ There are two types of covalent bonds: polar covalent bonds, where electrons are shared unequally, and nonpolar covalent bonds, where electrons are shared equally.
- 💡 The difference in electronegativity between two bonded atoms determines if a covalent bond is polar or nonpolar, with a difference of 0.5 or more indicating a polar bond.
- 🔍 Electronegativity values can be found in tables, and slight variations in these values can change the classification of a bond.
- 🤔 To determine the type of bond, consider the elements' positions in the periodic table and their electronegativity values.
- 🧫 Magnesium oxide (MgO) is an example of an ionic compound, composed of a metal (Mg) and a non-metal (O).
- 🌐 In practice problems, identify the type of bond by looking at the elements involved and their electronegativity differences, if applicable.
Q & A
What is the fundamental difference between ionic and covalent bonding?
-Ionic bonding involves the transfer of electrons from one atom to another, resulting in the formation of ions, whereas covalent bonding involves the sharing of electrons between atoms.
Which elements typically form ionic bonds, and why?
-Metals and non-metals typically form ionic bonds because metals tend to lose electrons to achieve a stable electron configuration, forming positive ions, while non-metals tend to gain electrons to achieve stability, forming negative ions.
How does the periodic table help in predicting the type of bonding between elements?
-Elements on the left side of the periodic table (metals) tend to form ionic bonds with elements on the right side (non-metals). Within groups of the same element, nonpolar covalent bonds are likely to form, while differences in electronegativity between different elements can lead to polar covalent bonds.
What is the role of valence electrons in ionic bonding?
-Valence electrons are the outermost electrons of an atom and play a crucial role in ionic bonding. Metals, having fewer valence electrons, tend to lose them to form positive ions, while non-metals with more spaces in their valence shell tend to gain electrons to form negative ions.
How does the concept of electronegativity influence the nature of covalent bonds?
-Electronegativity is the ability of an atom to attract electrons in a bond. In a covalent bond, if the electronegativity difference between the two atoms is small, the electrons are shared equally, resulting in a nonpolar covalent bond. If the difference is significant, the electrons are shared unequally, leading to a polar covalent bond.
What is a dipole, and how is it related to polar covalent bonds?
-A dipole is a separation of charge within a molecule, resulting in a molecule having a positive and a negative side. This occurs in polar covalent bonds when there is an unequal sharing of electrons due to a difference in electronegativity between the atoms involved.
How can you determine if a bond between two nonmetals is polar or nonpolar covalent?
-If two nonmetals are of the same element, the bond is nonpolar covalent because the electrons are shared equally. If they are different elements, you calculate the electronegativity difference. If the difference is 0.5 or greater, the bond is polar covalent; if it's less than 0.5, it's nonpolar covalent.
What is the electronegativity value difference that generally indicates a polar covalent bond?
-A difference in electronegativity values between the two bonding atoms of 0.5 or greater typically indicates a polar covalent bond.
Why is the bond between hydrogen and fluorine considered polar covalent?
-The bond between hydrogen and fluorine is considered polar covalent due to the significant difference in electronegativity between the two elements, with fluorine being more electronegative and pulling the shared electrons towards itself, resulting in a partial negative charge on fluorine and a partial positive charge on hydrogen.
How does the structure of the periodic table help in identifying likely ionic compounds?
-The periodic table can be used to identify likely ionic compounds by recognizing that metals, found on the left side, tend to form positive ions by losing electrons, while nonmetals, found on the right side, tend to form negative ions by gaining electrons.
What is the significance of the octet rule in understanding covalent bonding?
-The octet rule states that atoms tend to form bonds in such a way that they have eight electrons in their valence shell, giving them the same electron configuration as noble gases. This rule is significant for understanding how covalent bonds form, as atoms will share, gain, or lose electrons to achieve this stable configuration.
Why is the bond between two chlorine atoms considered nonpolar covalent?
-The bond between two chlorine atoms is considered nonpolar covalent because they are of the same element and share their electrons equally, resulting in a balanced distribution of charge with no separation of charge or dipole moment.
Outlines
🔬 Ionic and Covalent Bonding Basics
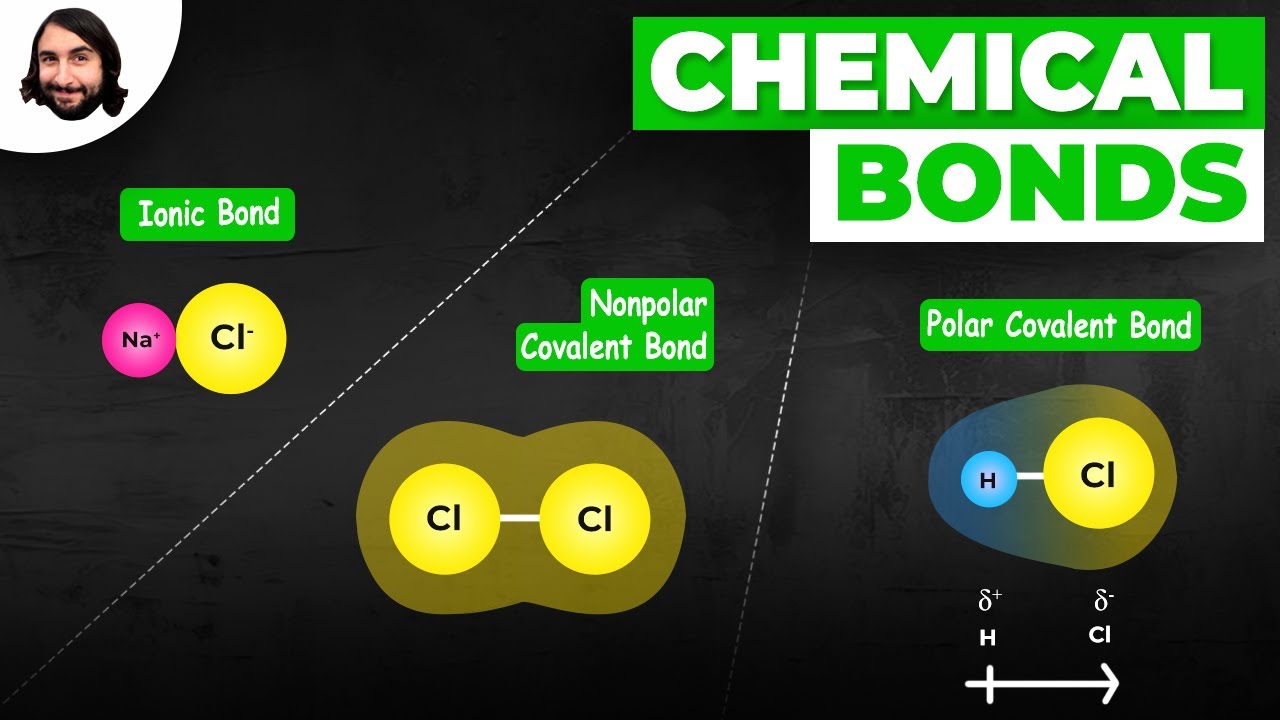
This paragraph introduces the concepts of ionic and covalent bonding. Ionic bonding involves the transfer of electrons from one element to another, resulting in the formation of ions. Covalent bonding, on the other hand, involves the sharing of electrons. The example of sodium (Na) and chlorine (Cl) is used to illustrate ionic bonding, where sodium donates an electron to chlorine, forming positively charged sodium ions and negatively charged chloride ions. These ions are attracted to each other due to opposite charges, creating an ionic bond. The paragraph also touches on the principles of electrostatic forces in ionic bonds and sets the stage for the discussion of covalent bonds, including hydrogen's need to share electrons to achieve a stable electron configuration.
🤝 Covalent Bonding: Polar and Nonpolar
The second paragraph delves into covalent bonding, explaining the difference between polar and nonpolar covalent bonds. It uses the example of two hydrogen atoms sharing electrons equally to form a nonpolar covalent bond. In contrast, the bond between hydrogen and fluorine is polar because fluorine, being more electronegative, pulls the shared electrons towards itself, resulting in a partial negative charge on fluorine and a partial positive charge on hydrogen. This creates a dipole and a polarized object. The concept of electronegativity is introduced as the ability of an atom to attract electrons, with fluorine being particularly effective. The paragraph concludes with a method to classify bonds as ionic, polar covalent, or non-polar covalent based on the elements involved and their positions in the periodic table.
🧠 Practice Problems: Classifying Bonds
The final paragraph presents practice problems to classify various bonds as ionic, polar covalent, or non-polar covalent. It provides a step-by-step approach, starting with identifying if a bond is ionic by checking for a metal and non-metal combination, as seen in magnesium oxide (MgO). For non-metal combinations, such as two chlorine atoms, the bond is nonpolar covalent due to equal sharing of electrons. In cases involving different elements, like sodium fluoride (NaF) and hydrogen bromide (HBr), the paragraph explains the need to calculate the electronegativity difference to determine if the bond is polar or nonpolar covalent. It also mentions the use of an electronegativity table for these calculations and provides specific values for hydrogen and bromine to illustrate the process. The paragraph concludes with additional examples, reinforcing the concepts learned and offering a comprehensive understanding of chemical bonding.
Mindmap
Keywords
💡Ionic Bonding
💡Covalent Bonding
💡Valence Electrons
💡Electronegativity
💡Polar Covalent Bond
💡Nonpolar Covalent Bond
💡Metals and Nonmetals
💡Electron Sharing
💡Dipole
💡Octet Rule
💡Electronegativity Difference
Highlights
Ionic bonding involves the transfer of electrons from one element to another, resulting in the formation of ions.
Sodium, a metal, loses an electron to form a positively charged ion, while chlorine, a non-metal, gains an electron to form a negatively charged ion.
Opposite charges attract, creating an ionic bond through electrostatic forces between the ions.
Covalent bonding involves the sharing of electrons between atoms.
Hydrogen atoms share their single valence electron to achieve a stable electron configuration.
Covalent bonds can be polar or nonpolar, depending on the equal or unequal sharing of electrons.
A nonpolar covalent bond, like the one between two hydrogen atoms, involves equal sharing of electrons.
A polar covalent bond occurs when there is an unequal sharing of electrons, as seen in the bond between hydrogen and fluorine.
Electronegativity differences determine whether a covalent bond is polar or nonpolar, with a difference of 0.5 or more indicating polarity.
Magnesium oxide (MgO) is an example of an ionic compound, consisting of a metal and a non-metal.
Two chlorine atoms will form a nonpolar covalent bond because they are the same type of element and share electrons equally.
Sodium fluoride (NaF) contains ionic bonding due to the metal (sodium) and non-metal (fluorine) elements involved.
The bond between hydrogen and bromine (HBr) is polar covalent, with an electronegativity difference of 0.7.
Iodine monobromide has a relatively nonpolar covalent bond due to the small electronegativity difference of 0.3 between iodine and bromine.
Carbon-hydrogen bonds are generally nonpolar covalent, with an electronegativity difference of 0.4.
Hydrogen bonds, such as the OH bond, are a special case of polar covalent bonding due to the high electronegativity difference.
Calcium sulfide is an ionic compound, as calcium is a metal and sulfur is a non-metal.
Identifying the type of chemical bond can often be deduced by the position of elements in the periodic table and their electronegativity values.
Transcripts
Browse More Related Video

Polar and Nonpolar Covalent Bonds
The Chemical Bond: Covalent vs. Ionic and Polar vs. Nonpolar

Types of Chemical Bonds - AP Chem Unit 2, Topic 1

Atomic Hook-Ups - Types of Chemical Bonds: Crash Course Chemistry #22

Ionic, covalent, and metallic bonds | Chemical bonds | Chemistry | Khan Academy

ATI TEAS 7 I Chemical Bonds I Chemistry I
5.0 / 5 (0 votes)
Thanks for rating: