Some Basic Concepts of Chemistry | Class 11 | Full Chapter
TLDRThis transcript outlines the foundational concepts of chemistry, including the distinction between pure and impure substances, properties of metals, physical quantities, and the International System of Units. It delves into scientific notation, significant figures, accuracy vs. precision, and dimensional analysis. Furthermore, it explains the laws of chemical combination, the mole concept, Avogadro's number, and the concepts of molarity and molality, providing essential knowledge for understanding chemistry.
Takeaways
- 📌 Matter is categorized into pure substances (e.g., iron sheet, sodium chloride) and impure substances (e.g., salt solution, salad) based on the uniformity of particle types.
- 🔍 Elements and compounds are considered pure substances because they consist of a single type of particle, whereas mixtures are impure due to the presence of different particle types.
- 🏃♂️ Physical properties of metals can be observed without changing the substance's identity, and include mass, volume, shape, color, and length, as well as melting point, boiling point, evaporation, sublimation, and condensation.
- 🌟 Chemical properties of a substance are observed when it undergoes a state change, reacting with acids, bases, water, or air, and include reaction to chemicals.
- 📈 Physical quantities are measurable entities like length and time, which are categorized into base physical quantities and derived physical quantities, with the International System of Units (SI) providing standardized units for measurement.
- 🔢 Prefixes are mnemonics added to units to denote multiples or fractions, with common ones including kilo (thousand), milli (thousandth), micro (millionth), and nano (billionth).
- 📊 Scientific notation is a method of expressing very large or very small numbers in the form of a × 10^n, where 1 ≤ |a| < 10 and n is an integer.
- 🔍 Significant figures refer to the certain and important digits in a measurement, determined by starting from the first non-zero digit and counting all subsequent digits.
- 🎯 Accuracy in measurements is about how close the result is to the actual value, while precision is about the closeness of multiple measurements to each other.
- 🔄 Dimensional analysis is the process of converting one set of units to another, such as from meters to kilometers or from Celsius to Kelvin.
- 🌐 Laws of chemical combination include the conservation of mass, definite proportion, multiple proportion, Charles's law (pressure and temperature relationship), and Avogadro's law (volume and number of moles relationship).
- 🧪 Molarity (m) is the number of moles of solute per liter of solution, disregarding the solvent, while molality (m) is the number of moles of solute per kilogram of solvent.
Q & A
What is the main difference between pure substances and impure substances?
-Pure substances consist of a single type of particle with identical properties, such as an iron sheet or sodium chloride. Impure substances, on the other hand, contain different types of particles with different properties, like a salt solution or a salad.
Why are elements and compounds considered pure substances?
-Elements and compounds are considered pure substances because they are composed of the same type of particles. For example, iron, gold, and sodium chloride are all made up of one type of particle, which gives them uniform properties throughout.
What are some examples of mixtures that are considered impure substances?
-Examples of mixtures that are impure substances include air, seawater, salt solutions, suspensions, colloids, and alloys like steel. These mixtures contain different types of particles with varying properties.
How can you remember the physical properties of metals using a mnemonic?
-A mnemonic for remembering the physical properties of metals is 'moving vent should come late', where 'm' stands for mass, 'v' for volume, 's' for shape, 'c' for color, 'l' for length, and 'e' for other physical changes like melting point and boiling point.
What are base physical quantities and which seven quantities are considered as such?
-Base physical quantities are the fundamental quantities from which other physical quantities are derived. The seven base physical quantities are length, mass, time, temperature, amount of substance, light intensity, and electric current.
What is the International System of Units (SI) and how can you remember the seven base units?
-The International System of Units (SI) is a globally recognized system of measurement established in 1960 for seven base physical quantities. To remember the base units, you can use the mnemonic 'lisa, mem turns to a left age' where 'l' stands for length (meter), 'm' for mass (kilogram), 't' for time (second), 'a' for temperature (kelvin), 's' for amount of substance (mole), 'l' for light intensity (candela), and 'e' for electric current (ampere).
What is the difference between mass and weight?
-Mass is the amount of matter present in an object and remains constant regardless of location, while weight is the force exerted on an object by gravity. The SI unit for mass is the kilogram (kg), and for weight, it is the newton (N).
How can you remember the prefixes used to indicate multiples or fractions in units?
-To remember the prefixes, you can use the mnemonic 'dead has keeped my great trach' for the positive prefixes where 'deca' (da) is 10^1, 'hecto' (h) is 10^2, 'kilo' (k) is 10^3, 'mega' (m) is 10^6, and 'tera' (t) is 10^12. For the negative prefixes, use 'the trach that can make me nice person' where 'desi' (d) is 10^-1, 'centi' (c) is 10^-2, 'milli' (m) is 10^-3, 'micro' (μ) is 10^-6, 'nano' (n) is 10^-9, and 'pico' (p) is 10^-12.
What is scientific notation and why is it used?
-Scientific notation is a way of writing very large or very small numbers by expressing them as a product of a number between 1 and 10 and a power of 10. It is used to save space and time when writing numbers that have many digits, making them more manageable and easier to read.
How can you determine significant figures in a number?
-To determine significant figures, start with the first non-zero digit from the left and count all subsequent digits, including zeros if they are between non-zero digits or to the right of a decimal point in the case of decimal numbers. All these digits are considered significant.
What is the difference between accuracy and precision in measurements?
-Accuracy refers to how close a measurement is to the actual value, while precision indicates how close multiple measurements are to each other. A measurement can be precise but not accurate if the measurements are consistently wrong but close to each other. Conversely, a measurement can be accurate but not precise if it is correct but varies significantly between multiple measurements.
What are the five laws of chemical combination and what do they describe?
-The five laws of chemical combination describe the basic rules of how atoms and molecules combine. They include the Law of Conservation of Mass (mass is neither created nor destroyed in a chemical reaction), the Law of Definite Proportion (chemical substances have elements in a fixed mass ratio), the Law of Multiple Proportion (the ratio of masses in different compounds can be expressed as whole numbers), Gay-Lussac's Law (pressure is directly proportional to temperature), and Avogadro's Law (volume is directly proportional to the number of moles or mass of a gas at constant temperature and pressure).
What is the concept of mole and Avogadro's number in chemistry?
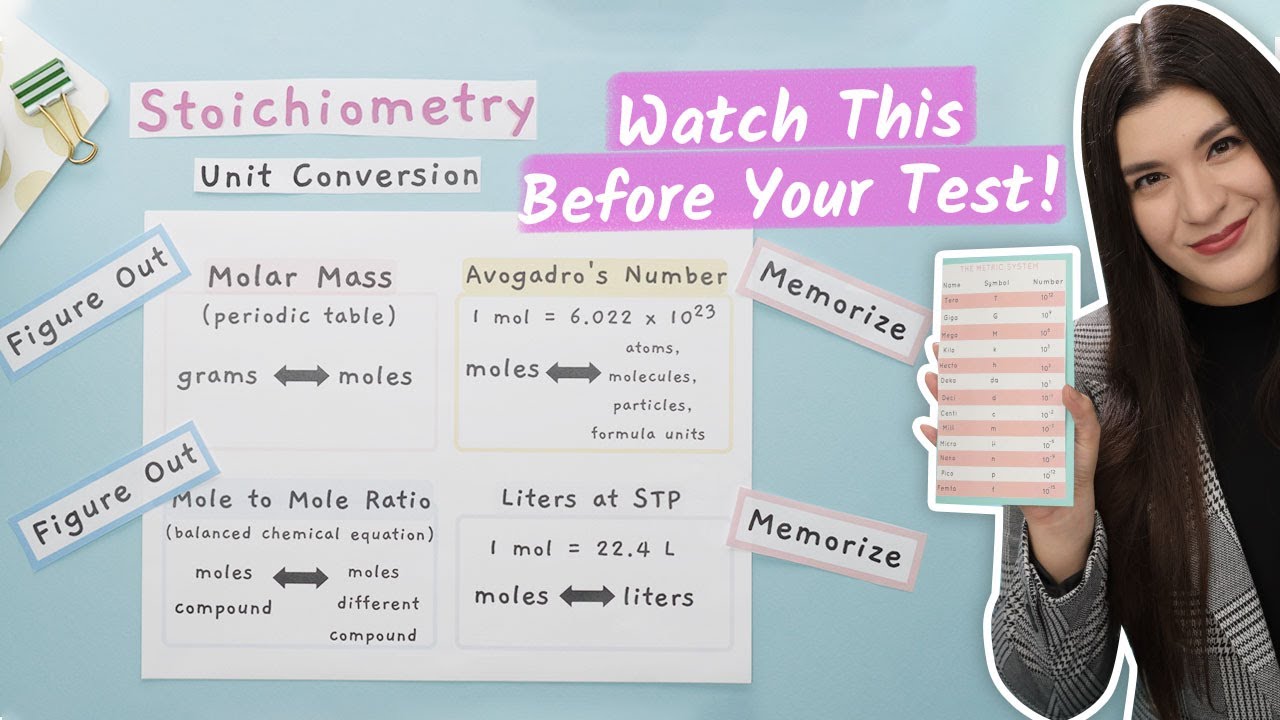
-A mole is a unit that represents the molar mass of an atom, molecule, or formula unit, which contains Avogadro's number of particles, approximately 6.023 x 10^23. Avogadro's number is the number of atoms in 12 grams of carbon-12, and it is used to relate the amount of substance to its mass and volume in chemistry.
How are molarity and molality different from each other?
-Molarity is the number of moles of solute dissolved in one liter of solution and does not consider the solvent's volume. Molality, on the other hand, is the number of moles of solute dissolved in 1 kg of solvent and takes into account the mass of the solvent.
Outlines
📚 Basic Concepts of Chemistry
This paragraph introduces fundamental concepts in chemistry, focusing on the classification of matter into pure and impure substances. It explains that pure substances, such as an iron sheet and sodium chloride, are composed of identical particles and thus have uniform properties. The distinction between elements and compounds as pure substances is clarified, and examples are provided. The paragraph also touches on the concept of impure substances, like mixtures, which consist of different types of particles. The importance of understanding these classifications for exams is emphasized, and several mnemonic devices are introduced to aid in memorizing key concepts.
🏃♂️ Properties of Metals and Physical Quantities
This section delves into the physical and chemical properties of metals, highlighting how physical properties can be observed without altering a substance's identity. Mnemonic devices are used to remember physical properties of metals. The concept of chemical properties is introduced, explaining how they are observed when a substance undergoes a state change. The paragraph then transitions to discuss physical quantities, differentiating between base and derived quantities, and the International System of Units (SI). A mnemonic trick is provided to remember the SI base units. Additionally, the difference between mass and weight is clarified, emphasizing their units and significance.
🔢 Prefixes and Scientific Notation
This paragraph discusses the use of prefixes in indicating multiples or fractions of units, with a mnemonic trick provided for easy recall. Scientific notation is introduced as a method for writing very large or small numbers, with examples given to illustrate the concept. The importance of significant figures in measurements is explained, along with a simple trick for determining significant figures in both non-decimal and decimal numbers.
🎯 Accuracy and Precision
The concept of accuracy and precision in measurements is explored, with examples provided to distinguish between the two. Accuracy refers to how close a measurement is to the actual value, while precision relates to the closeness of multiple measurements to each other. A mnemonic trick is introduced to help differentiate between the two, and multiple-choice questions are provided to test understanding of the concepts.
📐 Dimensional Analysis and Chemical Combination Laws
This section introduces dimensional analysis, a method for converting units from one system to another, with examples given. It then outlines five fundamental laws of chemical combination: the Law of Conservation of Mass, the Law of Definite Proportion, the Law of Multiple Proportion, Gay-Lussac's Law, and Avogadro's Law. Each law is explained with examples to illustrate their significance in understanding how atoms and molecules combine in chemical reactions.
🥚 Mole and Avogadro's Number, Molarity and Molality
The concept of the mole and Avogadro's number is introduced, relating to everyday quantities like a dozen eggs. The relative atomic mass and molar mass are explained, and the significance of Avogadro's number in understanding the number of particles in a mole is highlighted. The paragraph concludes with an explanation of molarity and molality, differentiating between the two and providing examples to illustrate their calculation and application.
Mindmap
Keywords
💡Pure substances
💡Impure substances
💡Physical properties
💡Chemical properties
💡Base physical quantities
💡Derived physical quantities
💡Significant figures
💡Molarity and Molality
💡Laws of chemical combination
💡Dimensional analysis
Highlights
The basic concept of matter is introduced, distinguishing between pure substances (uniform particles) and impure substances (different particles).
Elements and compounds are categorized as pure substances because they consist of one type of particle.
Mixtures are considered impure substances due to the presence of different types of particles with varying properties.
Physical properties of metals can be observed without changing the substance's identity, such as mass, volume, shape, color, and length.
Chemical properties are observed when a substance undergoes a state change, reacting with acids, bases, water, or air.
Physical quantities are measurable attributes, divided into base physical quantities and derived physical quantities.
The International System of Units (SI) was established in 1960 for seven base physical quantities.
Prefixes are mnemonics added to units to denote multiples or fractions, such as kilo, milli, micro, nano, and pico.
Scientific notation is a method of writing very large or small numbers in a space-efficient manner.
Significant figures are the certain and important digits in any measurement, identified by specific counting methods.
Accuracy and precision are defined, with accuracy referring to closeness to the actual value and precision to the closeness of multiple measurements to each other.
Dimensional analysis is the process of converting one set of units to another, as demonstrated with meter to kilometer and degree centigrade to kelvin.
The law of conservation of mass states that mass cannot be created or destroyed in a chemical reaction.
The law of definite proportion indicates that elements in chemical substances are present in a fixed mass ratio.
The law of multiple proportions explains that the ratio of masses in compounds can be expressed as whole numbers.
Gay-Lussac's law relates the pressure of a gas to its temperature, stating that they are directly proportional.
Avogadro's law associates the volume of a gas with the number of moles or mass at constant temperature and pressure.
Mole and Avogadro's number are introduced, with one mole containing 6.023 x 10^23 particles.
Molarity is defined as the number of moles of solute in one liter of solution, excluding the solvent.
Molality is the number of moles of solute in 1 kg of solvent, taking the solvent's mass into account.
Transcripts
Browse More Related Video

Unit Conversion & Significant Figures: Crash Course Chemistry #2

College Physics 1: Lecture 3 - Significant Figures and Scientific Notation

02 - What is Avogadro's Number & the Mole in Chemistry? Part 1

12 Chemistry Questions

Avogadro's Number, The Mole, Grams, Atoms, Molar Mass Calculations - Introduction
How to Convert Units in Chemistry
5.0 / 5 (0 votes)
Thanks for rating: