12 Chemistry Questions
TLDRIn this educational chemistry video, the host guides viewers through various chemistry concepts, including electronegativity differences predicting bond types, entropy changes in reactions, temperature conversions, scientific notation, and density calculations. The script also covers significant figures, precision vs. accuracy, the law of multiple proportions, and the calculation of average atomic mass. It concludes with a call to action for viewers to access pharmacology courses and chemistry notes on the host's website for further learning.
Takeaways
- 🧪 The video is part of a chemistry quick review series, aiming to test and improve viewers' understanding of chemistry concepts.
- 🔢 It explains how to determine the type of bond in sodium chloride (NaCl) by calculating the difference in electronegativity between sodium and chloride, concluding it's an ionic bond due to a difference greater than 1.7.
- 📈 The script discusses how an increase in the number of gas particles in a chemical reaction raises the entropy of the system, as gases represent a higher disorder compared to solids and liquids.
- K The video provides a method to convert temperatures from Celsius and Fahrenheit to the Kelvin scale, emphasizing the total temperature calculation of two substances.
- 🔑 It highlights the importance of scientific notation, explaining the correct format and the process of expressing numbers in a way that they fall between 1 and 10.
- 📏 The script covers the concept of density, demonstrating its calculation with an example of a silver cube and its mass and volume.
- 💧 The video explains how to find the volume of water displaced by an object, which is then used to calculate the object's density, using the example of a silver cube in water.
- 🔢 The importance of significant figures in scientific measurements is discussed, with an example of rounding a number to four significant figures.
- 🎯 The difference between precision and accuracy is clarified, using the analogy of an archer's consistency in hitting the target despite the distance from the bullseye.
- 🔬 The script introduces the law of multiple proportions to determine if two compounds are identical or different based on their mass ratios of elements.
- 🍎 It demonstrates how to calculate the average atomic mass of an element with isotopes, using the example of boron with different percentages of isotopes.
- 🧬 The molecular and empirical formulas of a compound are explained, using galactose as an example, and the difference between the two is highlighted.
- ⚛ The video concludes with a question about the ion with a specific atomic number, mass number, and charge, prompting viewers to identify the element and its ionic notation.
Q & A
What is the predicted type of bond in sodium chloride (NaCl) based on electronegativity values?
-The predicted type of bond in sodium chloride is ionic. This is because the difference in electronegativity between sodium (0.93) and chloride (3.16) is 2.23, which is greater than 1.7, indicating a strong tendency for ionic bonding due to the significant electronegativity difference.
How does an increase in the number of gas particles in a system affect the entropy of the system?
-An increase in the number of gas particles in a system raises the entropy. This is because entropy is a measure of disorder, and gases are less orderly than solids or liquids, so increasing the number of gas particles increases disorder and thus entropy.
What is the total temperature of a system when the temperature of A is 36.85 degrees Celsius and the temperature of B is 2.03 degrees Fahrenheit, when measured on the Kelvin scale?
-The total temperature of A and B on the Kelvin scale is 566.5 Kelvin. A's temperature in Kelvin is 310 K (36.85 C + 273.15), and B's temperature, after converting from Fahrenheit to Celsius (-16.65 C), is 256.5 K (-16.65 C + 273.15). Adding these gives the total temperature.
What is the proper scientific notation for the number 46000000?
-The proper scientific notation for the number 46000000 is 4.6 x 10^7. In scientific notation, a number is written as a product of a number between 1 and 10 and a power of 10, ensuring the first non-zero digit is to the right of the decimal point.
What is the density of a silver cube with a side length of 5 centimeters and a mass of 10 kilograms?
-The density of the silver cube is 8 x 10^3 kilograms per cubic meter. The volume of the cube is 125 cubic centimeters (5 cm * 5 cm * 5 cm), and converting this to cubic meters (0.000125 m^3), the density is calculated as mass divided by volume (10 kg / 0.000125 m^3).
What is the volume of water displaced by a silver cube with a mass of 60 grams when added to 30 mL of water, causing the water level to rise to 50 mL?
-The volume of water displaced by the silver cube is 20 mL, which is the difference between the initial water level (30 mL) and the final water level (50 mL) after the cube is added.
What is the density of the silver cube in the previous question, given that it displaced 20 mL of water?
-The density of the silver cube is 3 kilograms per liter. Since the cube displaced 20 mL (0.02 L) of water and has a mass of 60 grams, the density is calculated as mass divided by volume (60 g / 0.02 L).
How many significant figures are there in the number 0.05108?
-There are four significant figures in the number 0.05108. Significant figures start from the first non-zero digit and include all subsequent digits, including zeros that are between significant figures.
What is the difference between precision and accuracy in the context of archery?
-Precision refers to the consistency and reliability of measurements or, in the context of archery, the ability to hit the same spot repeatedly. Accuracy, on the other hand, refers to how close these hits are to the intended target or true value.
How can you determine if two compounds are different based on the law of multiple proportions?
-You can determine if two compounds are different by comparing the mass ratios of their constituent elements. If the mass ratios are in small whole numbers relative to each other, it suggests that the compounds obey the law of multiple proportions and are therefore different.
What is the average atomic mass for Boron if 80% of boron atoms have a mass of 11.0093 AMU and 20% have a mass of 10.0129 AMU?
-The average atomic mass for Boron is calculated using a weighted average, which in this case is (0.8 * 11.0093) + (0.2 * 10.0129) = 10.81 AMU. This takes into account the percentage of each isotope present in nature.
What is the molecular formula and empirical formula for a molecule of galactose with six carbon atoms, 12 hydrogen atoms, and six oxygen atoms?
-The molecular formula for galactose is C6H12O6, reflecting the exact number of atoms present. The empirical formula, which simplifies the ratio of atoms, is CH2O, obtained by dividing the number of each type of atom by the greatest common divisor, which is 6 in this case.
What is the symbol for the ion with a plus one charge, atomic number 19, and mass number 39?
-The symbol for the ion with a plus one charge, atomic number 19 (Potassium), and mass number 39 is written as 39K+, indicating the mass number above the element symbol and the charge as a superscript.
Outlines
🔬 Introduction to Chemistry Quick Review
Welcome to Medicosa's Perfect Net, where medicine makes perfect sense. This video features 12 chemistry questions with answers. The first question revisits the previous video’s topic on predicting the type of bond in sodium chloride based on electronegativity differences. By subtracting the electronegativity of sodium (0.93) from chloride (3.16), the difference of 2.23 indicates an ionic bond. The video further explains the types of bonds (ionic, polar covalent, non-polar covalent) based on the electronegativity difference.
🔢 Entropy and Temperature Calculations
The next question discusses entropy changes in a system when the number of gas particles increases, leading to higher disorder and thus higher entropy. Another problem involves converting temperatures from Celsius and Fahrenheit to Kelvin. A's temperature (36.85°C) converts to 310K, and B's temperature (2.03°F) converts to -16.65°C and then to 256.5K. Adding these gives a total of 566.5K.
📊 Scientific Notation and Density Calculations
This section covers scientific notation, emphasizing that the coefficient (M) must be between 1 and 10. The number 4.6 x 10^7 is used as an example. Density calculation is also discussed using a silver cube with a side length of 5 cm and a mass of 10 kg. The video explains the process of converting units and calculating density (0.08 kg/cm³ or 8 x 10^4 kg/m³).
💧 Volume Displacement and Significant Figures
A practical example involves calculating the density of a silver cube based on volume displacement in water. The cube raises the water level from 30 to 50 mL, indicating a volume of 20 mL. With a mass of 60 g, the density is 3 g/mL. Additionally, the process of rounding a number to four significant figures is demonstrated, highlighting the rules for determining and rounding significant figures.
📏 Exact and Estimated Measurements
This paragraph discusses measurements that can be determined exactly, such as liters to milliliters, hours to minutes, and dozens of items. It contrasts these with measurements that are estimated, like distances and personal heights. The video also explains precision in measurements, using archery as an analogy to differentiate precision from accuracy.
🔢 Law of Multiple Proportions and Atomic Mass
The video illustrates the law of multiple proportions through a comparison of mass ratios in two compounds. It explains how to determine if compounds are identical or different by comparing these ratios. It also covers the calculation of the average atomic mass of boron, considering the natural occurrence percentages of boron isotopes.
🧬 Molecular and Empirical Formulas
This section explains how to derive molecular and empirical formulas. Using galactose as an example, the molecular formula is given as C6H12O6, and the empirical formula is derived as CH2O by simplifying the ratio. The video also covers predicting bond types in compounds, writing symbols for ions based on atomic and mass numbers, and identifying cations.
📚 Additional Resources and Conclusion
The final paragraph promotes additional resources such as pharmacology and chemistry courses available on the Medicosa website. It encourages viewers to subscribe to the YouTube channel for more educational videos and to join the membership program for access to premium content. The video concludes with a call to action to like, subscribe, and visit the website for further learning materials.
Mindmap
Keywords
💡Electronegativity
💡Ionic Bond
💡Polar Covalent Bond
💡Entropy
💡Scientific Notation
💡Density
💡Significant Figures
💡Law of Multiple Proportions
💡Average Atomic Mass
💡Molecular Formula
💡Empirical Formula
Highlights
Introduction to the 10th video in the chemistry quick review playlist.
Explanation of how to determine the type of bond in sodium chloride based on electronegativity differences.
Clarification on the difference between nonpolar covalent, polar covalent, and ionic bonds.
Understanding the relationship between the number of gas particles and entropy in a chemical reaction.
Conversion of Celsius and Fahrenheit temperatures to the Kelvin scale.
Demonstration of scientific notation and its importance in expressing large numbers.
Calculation of the density of a silver cube using mass and volume.
The concept of significant figures and how to round numbers to a specific count.
Determination of the density of a cube by measuring the displaced water volume.
Understanding the difference between precision and accuracy in measurements.
Application of the law of multiple proportions to determine if two compounds are identical.
Calculation of the average atomic mass of Boron using weighted averages.
Identification of the molecular and empirical formulas of a molecule of galactose.
Explanation of the type of bond formed between hydrogen and oxygen in hydrogen peroxide.
Writing the symbol for an ion with a specific atomic number, mass number, and charge.
Invitation to download the general pharmacology course for further study.
Promotion of the chemistry notes and membership program for premium content access.
Closing remarks emphasizing the importance of studying hard and staying happy.
Transcripts
Browse More Related Video

Some Basic Concepts of Chemistry | Class 11 | Full Chapter

2021 Live Review 1 | AP Chemistry | Strategies for Multiple-Choice Questions Without a Calculator
Intermediate Chemistry: Essential Terms and Concepts Explained | LearningEnglishPRO 🧪🔬
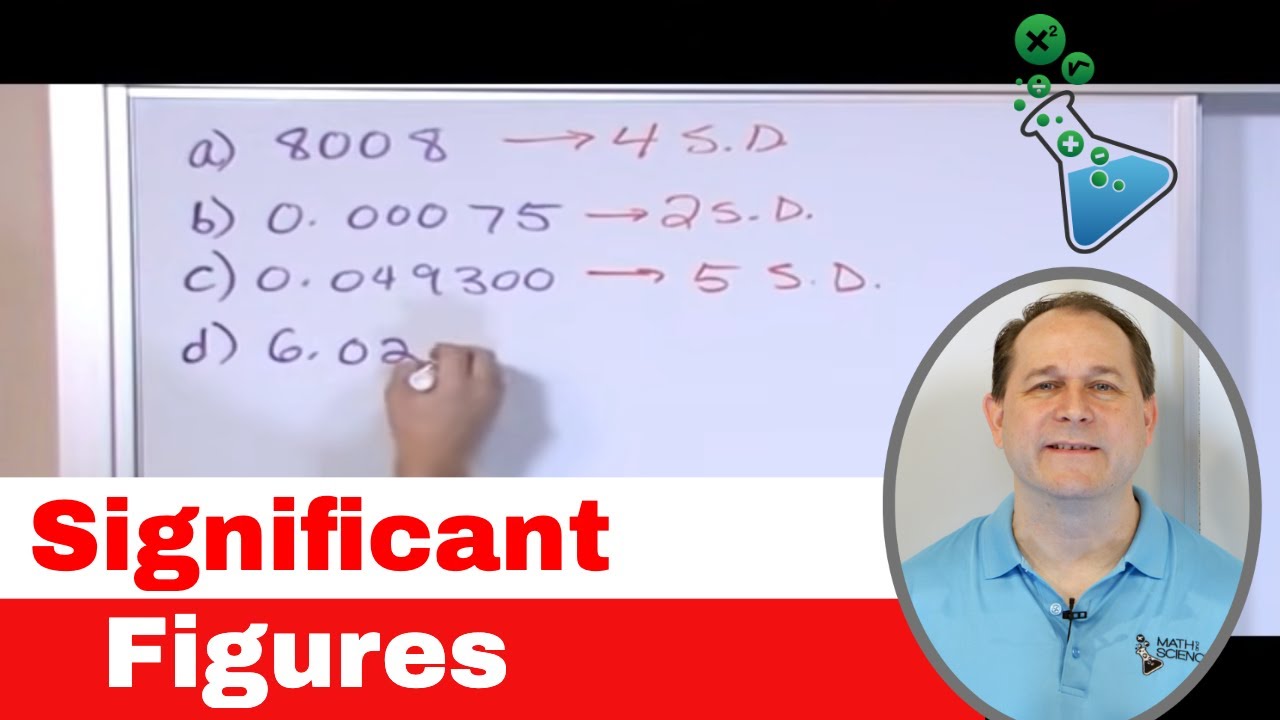
03 - Significant Figures Rules (Sig Fig Rules) for Calculations in Chemistry & Physics
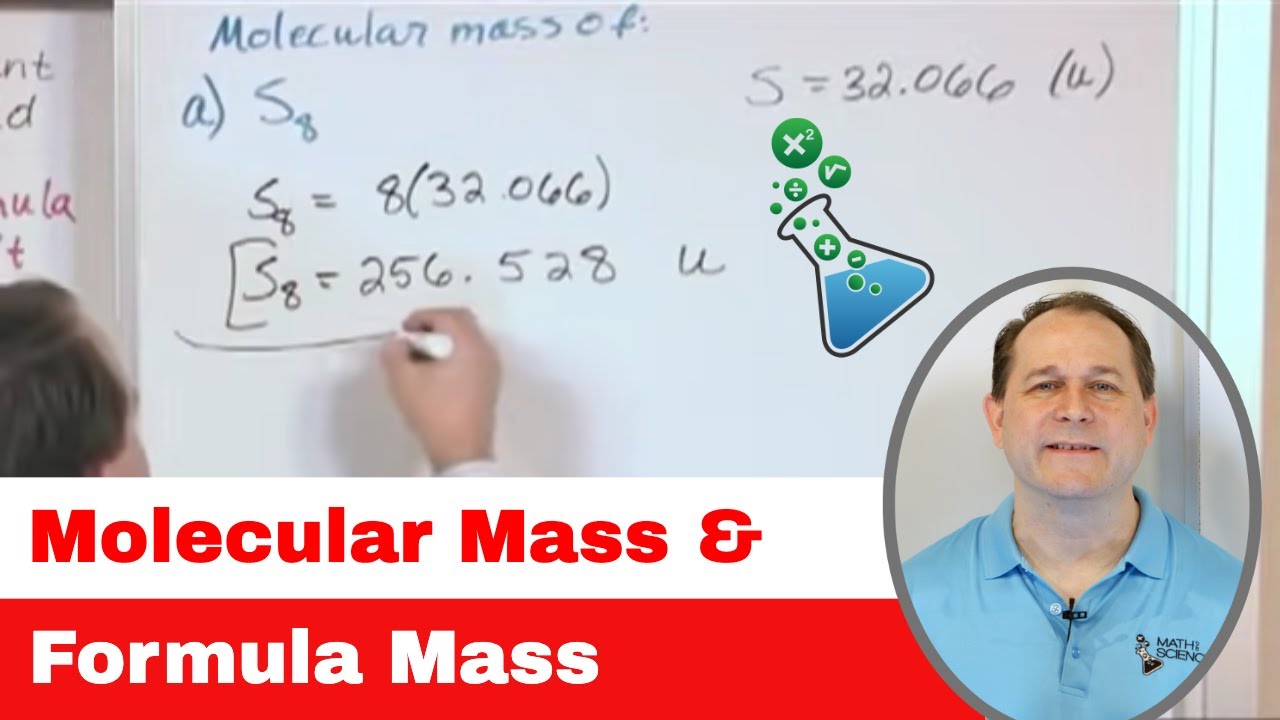
01 - Molecular Mass And Formula Mass - Learn the Formula Unit, Molecular Formula & Formula Mass
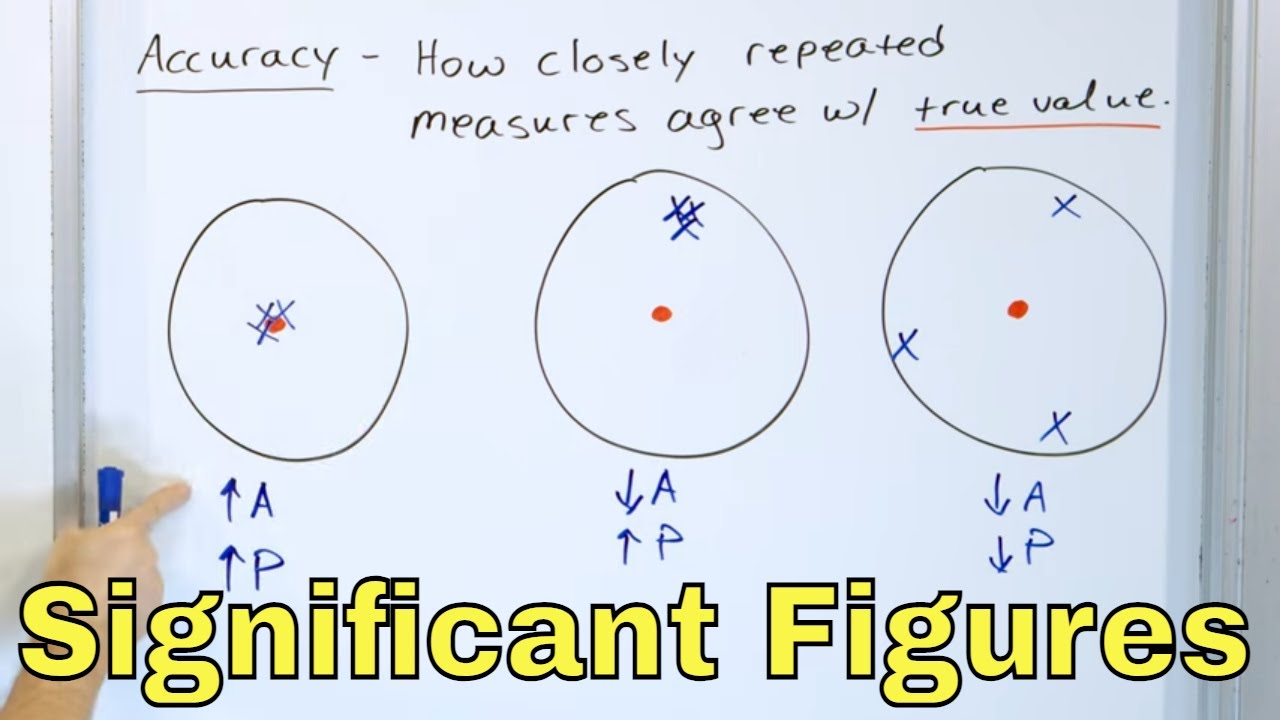
What are Significant Figures, Precision & Accuracy in Chemistry & Physics?
5.0 / 5 (0 votes)
Thanks for rating: