What is electricity? - Electricity Explained - (1)
TLDRThe video script delves into the fundamental concept of electricity, explaining it through the lens of atomic structure. It introduces the Bohr model to represent atoms and emphasizes the role of electrons in creating electric current. The script distinguishes between neutral atoms and ions based on electron count, and explains how the movement of electrons forms the basis of electrical charge and current. The importance of conductors and insulators in facilitating or impeding electron flow is highlighted, using everyday examples like static electricity and electrical wiring. The concept of an electrical circuit as a path for electron flow and the dangers of a short circuit are also covered, setting the stage for further exploration of electricity-related topics in future videos.
Takeaways
- 🔋 Electricity is fundamentally based on the movement of electrons, which are responsible for the flow of charge.
- 🔬 Atoms consist of a nucleus (protons and neutrons) and electrons orbiting around it, with the number of protons defining the element.
- ⚫️ Protons carry a positive charge and neutrons are neutral; electrons carry a negative charge.
- 💫 In a neutral state, the number of protons and electrons balance each other out, resulting in no net charge.
- 🔌 Atoms can become charged (ions) by gaining or losing electrons, leading to a positive or negative charge respectively.
- 🌐 The outermost shell of electrons (valence shell) influences an atom's reactivity, with a full shell indicating stability.
- 🚫 Insulators prevent the flow of electrons due to their valence shells being full, while conductors allow easy electron transfer.
- 🔍 The Bohr model, although simplified and not to scale, is used to explain the structure of atoms and the concept of electron shells.
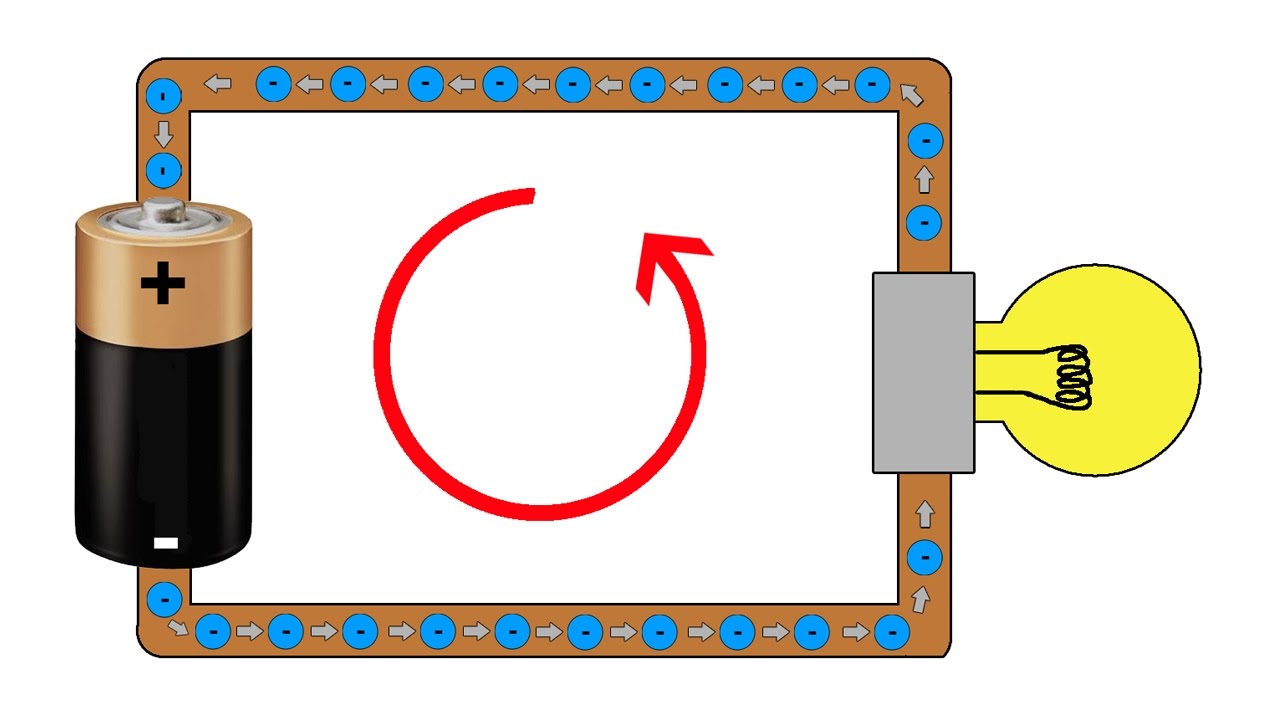
- 💡 An electrical circuit is a path that allows electrons to move from a negatively charged source to a positively charged attractor.
- 🔥 Short circuits occur when the path for electron flow is directly connected between the positive and negative poles of a power source, which can be dangerous.
- 🌐 The script also teases future content about power generation, resistance, voltage, batteries, fuses, motors, and transformers.
Q & A
What is electricity and why is it important to our civilization?
-Electricity is a form of energy arising from the movement of electric charges, typically electrons. It is vital to our civilization because it powers a wide range of technologies and devices that are essential for modern life, including lighting, heating, communication, transportation, and computing.
What is the Bohr model of an atom, and why is it used to explain electricity?
-The Bohr model of an atom is a simplified representation where electrons orbit the nucleus in certain paths or shells, similar to planets around the sun. It is used to explain electricity because it helps visualize the behavior of electrons, which are responsible for electric charge and current flow.
What defines the type of element in an atom?
-The type of element is defined by the number of protons in the nucleus of the atom. Each element has a unique atomic number, which corresponds to the number of protons it contains.
How does an atom become charged?
-An atom becomes charged when it either gains or loses electrons. If an atom has more electrons than protons, it becomes negatively charged, and if it has fewer electrons than protons, it becomes positively charged.
What is the role of valence electrons in an atom?
-Valence electrons, which are the electrons in the outermost shell of an atom, play a crucial role in chemical reactions and the reactivity of the atom. A full valence shell indicates a stable and less reactive atom, while an incomplete valence shell makes the atom more likely to engage in chemical bonding to achieve stability.
What are conductors and insulators, and how do they differ in terms of electron mobility?
-Conductors are materials that allow the flow of electric charge, meaning they have loosely bound valence electrons that can be easily transferred or lost. Insulators, on the other hand, have electrons that are more tightly bound, resulting in low electron mobility and preventing the flow of electric charge.
How does an electrical circuit work?
-An electrical circuit is a path that connects two points with a potential for charge imbalance, typically connecting a negatively charged point to a positively charged one. It allows the flow of electrons from a power source, through a conductor, to an attractor of electrons, creating a closed loop where energy is transferred and work is done.
What happens during a short circuit, and why is it dangerous?
-A short circuit occurs when the two poles of a power source are connected directly without any resistance in between. This allows the electrons to flow without any hindrance, leading to an instantaneous release of energy, often causing the wire to heat up dangerously and potentially leading to fires or damage to electrical devices.
What is a fuse, and why is it important in electrical systems?
-A fuse is a protective device in an electrical circuit that melts and breaks the circuit when the current exceeds a certain level, preventing potential damage or fire. It's important because it helps maintain safety by cutting off the electrical flow when it becomes too high.
How do batteries function in an electrical circuit?
-Batteries function as a source of electrons in an electrical circuit. They push out electrons from one end and attract them at the other, creating a flow of electric current. The movement of electrons from the negative to the positive terminal of the battery powers the circuit and allows devices to operate.
What are the colors used to represent the charge of atoms in the video?
-In the video, a positive charge, or the absence of electrons, is represented with red, a negative charge, or a surplus of electrons, is represented with blue, and a neutral charge, or a balance between electrons and protons, is represented by a blend of red and blue, which is purple.
What is the significance of the valence shell in determining the reactivity of an atom?
-The valence shell, which is the outermost shell of an atom, determines the reactivity of the atom because it houses the valence electrons that participate in chemical reactions and bonding. If the valence shell is full, the atom is generally stable and less reactive. If it's not full, the atom tends to be more reactive as it seeks to achieve a stable electron configuration.
Outlines
🔋 Understanding Electricity and Atomic Structure
This paragraph introduces the concept of electricity and its fundamental role in our civilization. It delves into the atomic level to explain the structure of atoms, using the Bohr model as a representation. The explanation highlights the importance of electrons in generating electricity and how their movement forms an electric current. The balance of protons and electrons in an atom results in a neutral charge, while imbalances result in positive or negative charges. The paragraph also touches on the concepts of ions, electron shells, and the reactivity of atoms based on their valence electrons.
🚶♂️ The Dynamics of Charging and Discharging
This section discusses the dynamics of charging and discharging through everyday examples, such as shuffling feet on a carpet. It explains how the loss of negatively charged electrons from the body to the carpet creates a charge imbalance. The distinction between conductors, like the human body, and insulators, like the carpet, is clarified. The paragraph further explains how touching a metal object can result in a discharge. The nature's tendency to seek a neutral charge equilibrium is highlighted, along with the roles of conductors and insulators in facilitating or impeding the flow of electrons. The concept of an electrical circuit and its importance in the flow of electricity is also discussed.
🔌 Exploring Conductors, Insulators, and Electrical Circuits
This paragraph explores the properties of conductors and insulators, using a simple wire as an example to illustrate their combined function in electrical systems. The copper core's conductivity and the plastic shell's insulating properties are explained. The paragraph details how electrons move through a conductor to create an electrical circuit, drawing parallels with marbles in a tube. It emphasizes the necessity of a continuous electrical circuit for the flow of electricity and the dangers of a short circuit. The role of fuses in preventing damage from excessive current is also mentioned, along with a teaser for upcoming videos on various electrical concepts and devices.
📢 Engaging with Educational Content
The final paragraph shifts from the technical explanation of electricity to engage viewers directly. It encourages subscription and notification for new content, promotes sharing the video with others who might appreciate it, and expresses gratitude for the support of the audience and Patreon contributors. The paragraph concludes with an invitation to explore more content and acknowledges the sources used in creating the video.
Mindmap
Keywords
💡Electricity
💡Atoms
💡Electrons
💡Protons
💡Electric Current
💡Conductors
💡Insulators
💡Valence Electrons
💡Electrical Circuit
💡Charge Imbalance
💡Ground State
Highlights
Electricity is fundamental to modern civilization, and understanding it requires delving into atomic structure.
Atoms, the basic units of matter, are too small to be seen without specialized equipment like a scanning tunnelling microscope.
The Bohr model, although not to scale, is a useful representation to explain the components of an atom, including protons, neutrons, and electrons.
Electrons orbiting the nucleus are responsible for electrical properties, moving in regions or clouds rather than fixed paths.
An atom's identity as a specific element is determined by the number of protons in its nucleus.
The number of electrons an atom has in relation to its protons dictates its electrical charge and reactivity.
In a neutral state, the positive and negative charges within an atom balance each other out, resulting in a net electric charge of zero.
Atoms can become ions by gaining or losing electrons, resulting in a net positive or negative charge.
The valence shell and its electrons determine an atom's reactivity, with a full outer shell indicating chemical stability.
Static electricity is a common example of charge imbalance, occurring when electrons are transferred between different materials.
Insulators and conductors play key roles in the flow of electricity; insulators retain electrons while conductors allow them to move freely.
A simple electrical circuit consists of a path connecting a charge source to a charge attractor, allowing for the flow of electrons.
Materials with high electron mobility, like metals, are conductors, whereas those with low mobility are insulators.
A short circuit occurs when the poles of a power source are connected directly, which can be dangerous due to uncontrolled electron flow and energy release.
Fuses are safety devices that cut off the electrical current when it becomes too high, preventing potential damage or fire.
Electrons are not consumed in the process of electricity; they are merely carriers of charge that move through a circuit.
The concept of an electrical circuit and the role of conductors and insulators are essential to understanding how electrical devices function.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: