What is Electric Charge and How Electricity Works | Electronics Basics #1
TLDRIn this informative video, Dan Dolkovsky from howtomechatronics.com explores the fundamental concepts of electric charge and electricity. Starting with the atomic level, the video explains the structure of an atom based on the Bohr model, highlighting the roles of protons, neutrons, and electrons. It then discusses how the movement of electrons forms electric current and the importance of conductors and insulators. The concept of charge conservation is introduced, along with the law of conservation of electric charge. The video concludes by touching on how electric current is measured in amperes and sets the stage for future discussions on Coulomb's law and electric fields.
Takeaways
- 🌟 Atoms are the fundamental building blocks of matter, consisting of a nucleus (neutrons and protons) and orbiting electrons.
- 🔋 The Bohr model is a simplified representation of the atom, useful for understanding its structure.
- ⚡ Electrons orbit the nucleus in shells, with each shell holding a specific number of electrons, determining the atom's reactivity.
- 🏎️ The outermost shell, or valence shell, influences an atom's tendency to form chemical bonds, with a full shell indicating stability.
- 🔌 Materials can be conductors (like metals) or insulators (like plastic), affecting the movement of electrons.
- 💥 Atoms are generally neutral, having equal numbers of protons and electrons, but can become charged by gaining or losing electrons.
- 🌐 Charge imbalance leads to the transfer of electrons, such as when dragging feet across a carpet, creating static electricity.
- 🔄 Nature seeks a neutral charge, and the law of conservation of electric charge states that charge cannot be created or destroyed, only transferred.
- 🔋 The unit of electric charge is the coulomb, and the charge of a single electron or proton is known as the elementary charge.
- 💡 Electric current is the flow of electrons in a circuit, from a power source's negative terminal to its positive terminal.
- 🌠 The next video will discuss Coulomb's law and the electric field, further exploring the principles of electricity.
Q & A
What is the basic building block of matter?
-The basic building block of matter is the atom.
What is the Bohr model of an atom?
-The Bohr model of an atom is a simplified model that places a nucleus at the center consisting of neutrons and protons, with electrons orbiting around it in circular shells.
What are the fundamental particles in an atom's nucleus?
-The fundamental particles in an atom's nucleus are neutrons, which have no charge, and protons, which have a positive charge.
How do electrons behave in relation to the nucleus?
-Electrons orbit around the nucleus in constant motion, with the lighter electrons circulating in shells or energy levels that can hold a specific number of electrons.
What determines the reactivity of an atom?
-The reactivity of an atom, or its tendency to form chemical bonds, is determined by the number of electrons in the outermost shell, known as the valence shell.
What are free electrons and how do they relate to materials' conductivity?
-Free electrons are loosely bound electrons in the valence shell that can move from one atom to another. The ease with which electrons move depends on the material; conductors like metals allow free movement, while insulators like plastic or glass limit electron movement.
What is the significance of an atom having a neutral charge?
-An atom with a neutral charge has the same number of electrons as protons, resulting in a net electric charge equal to zero, which is the lowest possible energy level or the ground state of the atom.
How can an atom's charge be changed?
-An atom's charge can be changed by causing it to gain or lose electrons. If an atom gains electrons, it becomes negatively charged, and if it loses electrons, it becomes positively charged, forming ions.
What is the law of conservation of electric charge?
-The law of conservation of electric charge states that you cannot create a net electric charge. Instead, charge can only move from one place to another, and the overall charge between objects remains constant.
What is the unit of electric charge?
-The unit of electric charge is the coulomb.
What is the flow of electrons in a circuit?
-The flow of electrons in a circuit forms an electric current. When a closed circuit is created with a power source, such as a battery, the voltage causes electrons to move in the same direction, creating a current.
How is electric current measured?
-Electric current is measured in amperes. One coulomb of charge passing through a wire over one second is equal to one ampere of current.
Outlines
🔋 Understanding Electric Charge and Atoms
This paragraph introduces the concept of electric charge and its fundamental role in understanding electricity. It begins by explaining that matter, including everything in the universe, is composed of atoms. The Bohr model of the atom is introduced as a simplified way to understand the atom's structure, highlighting the nucleus (containing neutral neutrons and positively charged protons) and negatively charged electrons orbiting around it. The number of neutrons, protons, and electrons defines the material, with the outermost electrons, known as valence electrons, determining the atom's reactivity and tendency to form chemical bonds. The paragraph also discusses how materials can be conductors or insulators based on their ability to allow electron movement. It concludes by explaining how atoms can gain or lose electrons to become ions and the law of conservation of electric charge, which states that electric charge can't be created or destroyed, only transferred.
💡 Electric Current and Circuits
The second paragraph delves into the formation of electric current through the flow of electrons. It describes how, within a copper wire, atoms facilitate the easy exchange of electrons, which can move in any direction. When a closed circuit is created by connecting the wire to a power source like a battery, the voltage causes electrons to move uniformly from one terminal to another. The addition of a light bulb in the circuit demonstrates that electrons must pass through it to reach the other terminal, producing light. The paragraph defines electric current (symbolized as 'i') and its unit of measurement, the ampere, which is one coulomb of charge passing through a wire in one second. The video concludes with a teaser for the next episode, which will cover Coulomb's law and the electric field.
Mindmap
Keywords
💡Electric Charge
💡Atom
💡Electrons
💡Nucleus
💡Valence Shell
💡Conductors
💡Insulators
💡Electric Current
💡Coulomb
💡Law of Conservation of Electric Charge
💡Coulomb's Law
Highlights
Understanding electricity begins with the atom, the basic building block of matter.
The Bohr model is a simplified representation to comprehend the atom's structure.
An atom consists of a nucleus with neutrons and protons, and electrons orbiting around it.
The number of neutrons, protons, and electrons defines the material's identity.
Electrons determine an atom's reactivity through their presence in the valence shell.
Materials can be conductors, allowing free electron movement, or insulators, limiting it.
Atoms are generally neutral, with equal numbers of electrons and protons, representing the ground state.
Charge imbalance occurs when atoms gain or lose electrons, resulting in positive or negative ions.
Static charge is built through electron transfer between surfaces, like dragging feet on a carpet.
Nature seeks a neutral charge equilibrium, leading to the law of conservation of electric charge.
The unit of electric charge is the coulomb, denoted by 'q'.
The charge of a single electron or proton is known as the elementary charge, 'e'.
Electron flow within a circuit, like a copper wire, constitutes an electric current.
A closed circuit with a power source directs electrons to move in a specific direction.
One coulomb of charge passing through a wire in one second equals one ampere of current.
Electric current is measured in amperes and represents the movement of charge over time.
The video introduces fundamental concepts of electricity and atomic structure.
The video explains the role of electrons, protons, and neutrons in determining an atom's properties.
The video discusses the importance of the valence shell in chemical reactivity.
The video covers the basics of conductors and insulators in the context of electricity.
The video touches on the concept of static electricity and its everyday occurrence.
The video provides an overview of the law of conservation of electric charge.
The video explains the concept of electric current and its relation to the movement of electrons.
The video concludes with a teaser for the next topic, Coulomb's law and electric field.
Transcripts
Browse More Related Video

What is electricity? - Electricity Explained - (1)
Electric Charge and Electric Fields
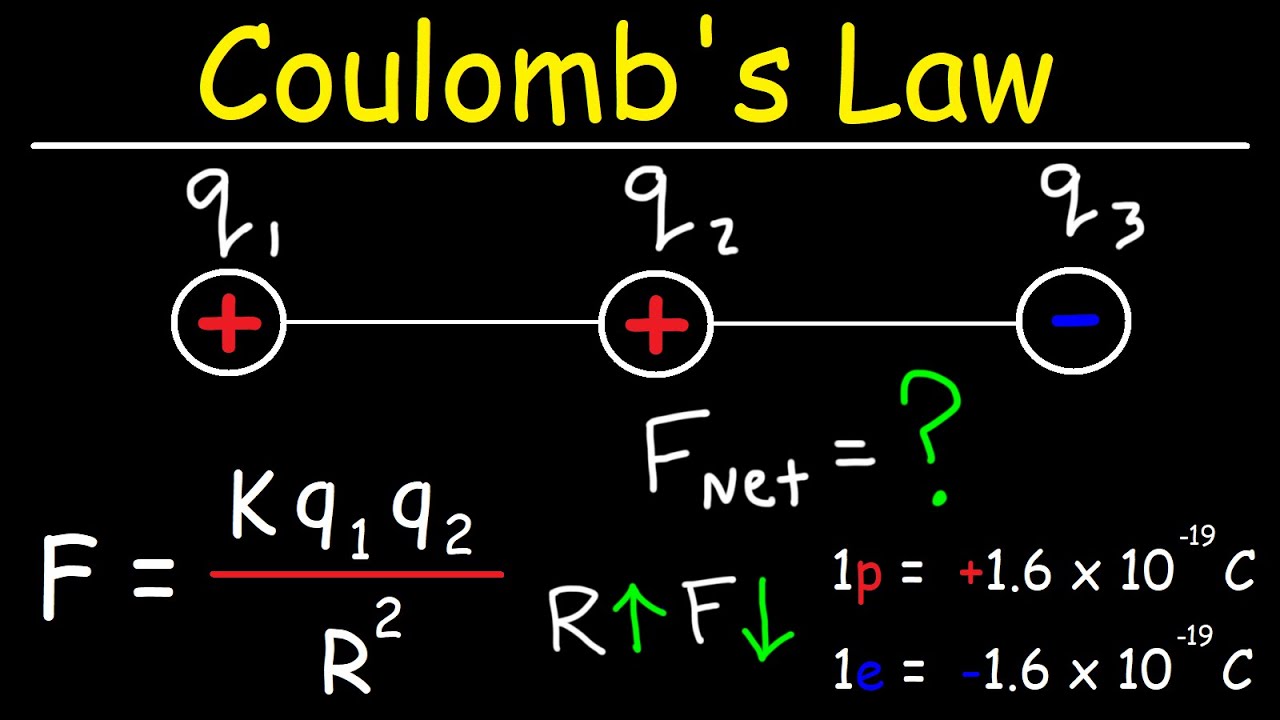
Coulomb's Law - Net Electric Force & Point Charges

AP Physics 1 Review of Charge and Circuit | Physics | Khan Academy

Electric Charges and Electric Fields - Review for AP Physics C: Electricity and Magnetism

What is Voltage?
5.0 / 5 (0 votes)
Thanks for rating: