How Many Carbons And Hydrogens Are In These Compounds (Organic Chemistry)
TLDRThis educational video script teaches viewers how to determine the number of carbons and hydrogens in line structures of organic compounds. The presenter guides through the process step-by-step, explaining the importance of carbon's valence electrons and how to count bonds to infer the presence of hydrogen atoms. The methodical approach involves counting carbons first, then deducing hydrogens based on the carbon's need for four bonds. The script provides a practical learning tool for beginners in organic chemistry, encouraging practice and understanding of molecular structures.
Takeaways
- 📚 The video teaches how to identify the number of carbons and hydrogens in line structures.
- 🔍 Each carbon atom is represented in the line structure, and counting them directly gives the total number of carbons.
- 🌐 Carbon has a valence electron count of four, which means it needs four bonds to complete its octet.
- 🔗 By counting the bonds around each carbon, you can determine which carbons are missing hydrogen bonds.
- 🚀 For carbons with four bonds, no hydrogens are present because they are fully bonded.
- 💡 Hydrogens are inferred from the remaining bonds needed for each carbon to complete its four bonds.
- 📈 The process involves counting bonds and subtracting them from the total needed (four) to find the number of hydrogens.
- 🔢 The video provides a step-by-step method to count carbons and hydrogens in different line structures.
- 🔬 Practice is emphasized as a key to mastering the technique for identifying carbons and hydrogens in organic chemistry.
- 🌟 The video encourages viewers to pause and try the exercise themselves to check their understanding.
Q & A
How many carbons are in the first compound discussed in the video?
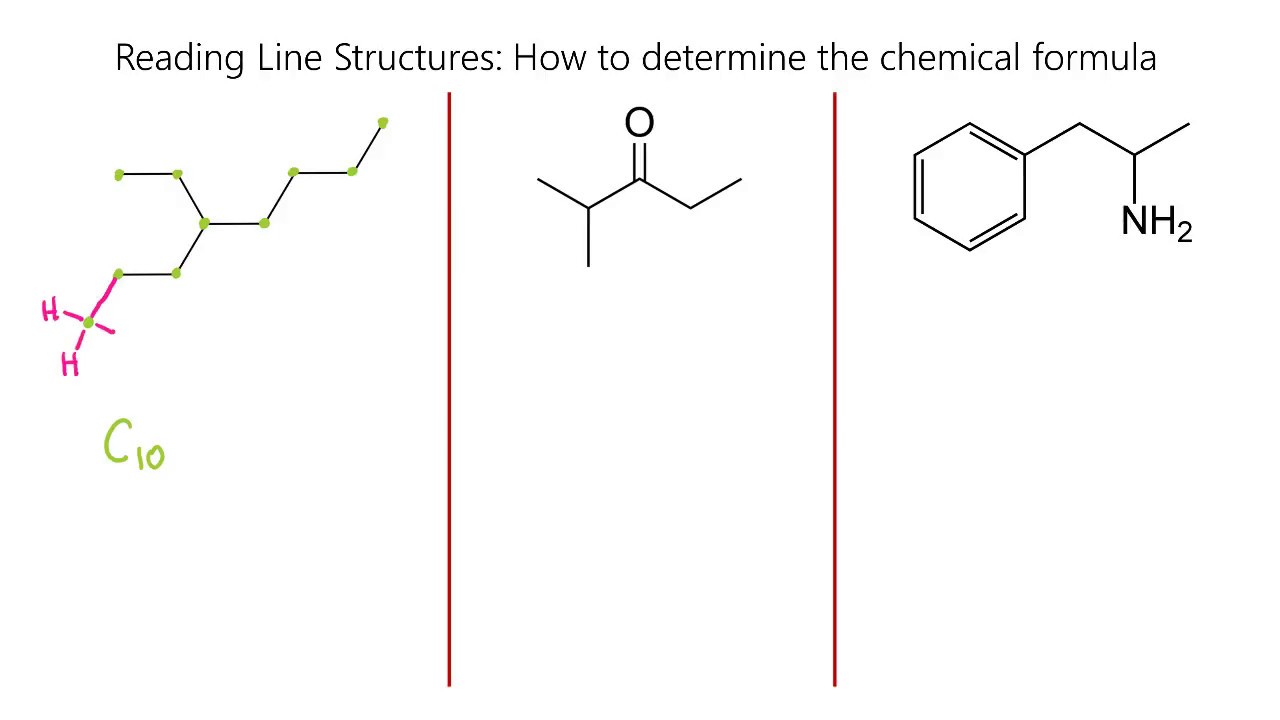
-There are nine carbons in the first compound discussed in the video.
What is the valence electron count for carbon, and how does it relate to its bonding needs?
-Carbon has four valence electrons and needs four more to complete its octet, which means it must have four bonds at all times.
Why are hydrogens not always explicitly shown in line structures?
-Hydrogens are not always shown in line structures because of the knowledge of bonding; it is understood that carbon needs to have four bonds, and the missing bonds are assumed to be hydrogens unless otherwise indicated.
How can you determine the number of hydrogens attached to a carbon atom in a line structure?
-You can determine the number of hydrogens by counting the bonds each carbon atom has. If a carbon atom does not have four bonds, the difference is made up by hydrogens.
What is the total number of hydrogens in the first compound after identifying all the bonds?
-After identifying all the bonds, the first compound has a total of 10 hydrogens.
How many carbons are there in the second compound discussed in the video?
-There are ten carbons in the second compound discussed in the video.
What is the total number of hydrogens in the second compound after analyzing the carbon bonds?
-After analyzing the carbon bonds, the second compound has a total of 16 hydrogens.
How many carbons are in the third compound that the viewer is encouraged to identify themselves?
-There are eleven carbons in the third compound that the viewer is encouraged to identify.
What is the total number of hydrogens in the third compound after counting all the bonds?
-After counting all the bonds, the third compound has a total of 22 hydrogens.
What is the purpose of the video script in terms of educating viewers about organic chemistry?
-The purpose of the video script is to educate viewers on how to identify the number of carbons and hydrogens in line structures, which is a fundamental skill in organic chemistry.
What advice does the video provide for beginners learning organic chemistry?
-The video advises beginners to practice step by step, starting with counting carbons and hydrogens in line structures, and assures them that with practice, it will become easier.
Outlines
🧪 Counting Carbons and Hydrogens in Line Structures
This paragraph introduces the process of identifying the number of carbons and hydrogens in chemical compounds represented by line structures. The speaker explains that each line represents a carbon atom and that by counting the bonds each carbon has, one can determine the number of hydrogens attached. The example given walks through counting the carbons (nine in total) and then deducing the number of hydrogens by considering the carbon's valence electrons and the need for each carbon to have four bonds. The process involves identifying incomplete bonds and adding the necessary hydrogens to complete them, resulting in a total of ten hydrogens.
🔍 Advanced Carbon and Hydrogen Counting in Organic Chemistry
The second paragraph continues the theme of counting carbons and hydrogens but with a more complex example. The speaker counts ten carbons in the compound and then systematically identifies the number of hydrogens each carbon atom is associated with, based on the number of bonds it has. The explanation includes the principle that carbon atoms need four bonds to satisfy their valence electron requirement. By the end of the paragraph, the speaker sums up the total number of hydrogens, which is sixteen, and encourages viewers to practice this method to become proficient in organic chemistry.
📚 Tips for Beginners in Organic Chemistry
The final paragraph offers advice for beginners in organic chemistry, emphasizing the importance of practice to master the skill of identifying carbons and hydrogens in line structures. The speaker suggests that while the counting process may seem tedious at first, with practice, it will become easier and more intuitive. The paragraph concludes with an invitation for viewers to like, subscribe, and follow the channel for more educational content on organic chemistry.
Mindmap
Keywords
💡Carbons
💡Hydrogens
💡Line Structures
💡Valence Electrons
💡Octet Rule
💡Bonding
💡Molecular Structure
💡Counting Bonds
💡Organic Chemistry
💡Practice
💡Like and Subscribe
Highlights
Introduction to identifying the number of carbons and hydrogens in line structures.
Method to count visible carbons in a compound by observing the structure.
Understanding that carbon has a valence electron count of four and needs four bonds to complete its octet.
Technique to determine the number of bonds each carbon contains and infer missing hydrogens.
Explanation of how to count hydrogens by identifying incomplete carbon bonds.
Step-by-step counting of hydrogens for a compound with nine carbons.
Final count of ten hydrogens for the first compound example.
Second example compound with ten carbons and the process of identifying hydrogens.
Counting hydrogens for each carbon in the second compound based on their bond count.
Final tally of sixteen hydrogens for the second compound.
Third example with an eleven-carbon compound and the method to determine hydrogens.
Detailed process of counting hydrogens for the third compound's carbons.
Final count of twenty-two hydrogens for the third compound.
Encouragement for viewers to practice and gain proficiency in organic chemistry.
Invitation to like and subscribe for more educational content on organic chemistry.
Transcripts
Browse More Related Video

How to Draw Skeletal Structure or Bond-Line Notation for Organic Molecules
Reading Skeletal Line Structures (Organic Chemistry), Part 1

2.2 Drawing Line Angle Structures (aka Bond Line Structures) | Organic Chemistry

Naming Cycloalkanes With Substituents, Cis & Trans, Bicyclo Alkane Nomenclature

General Chemistry Review for Organic Chemistry

Visualize & Name Organic Compounds in Organic Chemistry - [1-2-32]
5.0 / 5 (0 votes)
Thanks for rating: