Assigning R/S on Fischer Projections
TLDRIn this educational video, Professor Dave delves into the concept of Fischer projections, a method used to represent stereochemistry in molecules, particularly for carbohydrates. He explains the notation, emphasizing that horizontal lines imply wedge bonds and vertical lines imply dash bonds, reflecting the tetrahedral geometry of sp3 hybridized carbons. The video also covers the application of the Cahn-Ingold-Prelog priority rules to assign R/S configurations to stereocenters in Fischer projections, providing clear guidelines for students to correctly interpret and assign configurations, even when the lowest priority group is on a horizontal bond.
Takeaways
- 🧑🏫 The tutorial is presented by Professor Dave, focusing on Fischer projections, a method to represent stereochemistry in organic chemistry.
- 📚 Fischer projections are commonly used to depict the stereochemistry of simple sugars like monosaccharides, though they can be applied to other molecules as well.
- 🔍 In Fischer projections, horizontal lines represent wedge bonds, and vertical lines represent dash bonds, implying a three-dimensional structure.
- 🌐 It's important to understand that Fischer projections are a flat representation and do not depict the actual tetrahedral geometry of sp3 hybridized carbons.
- 🔄 To visualize the molecule, one can imagine a 'horseshoe' or 'rollercoaster' shape, where each successive bond moves further away from the viewer.
- 📝 When assigning R/S configuration using the Cahn-Ingold-Prelog (CIP) convention, the priority is given to substituents based on their atomic number.
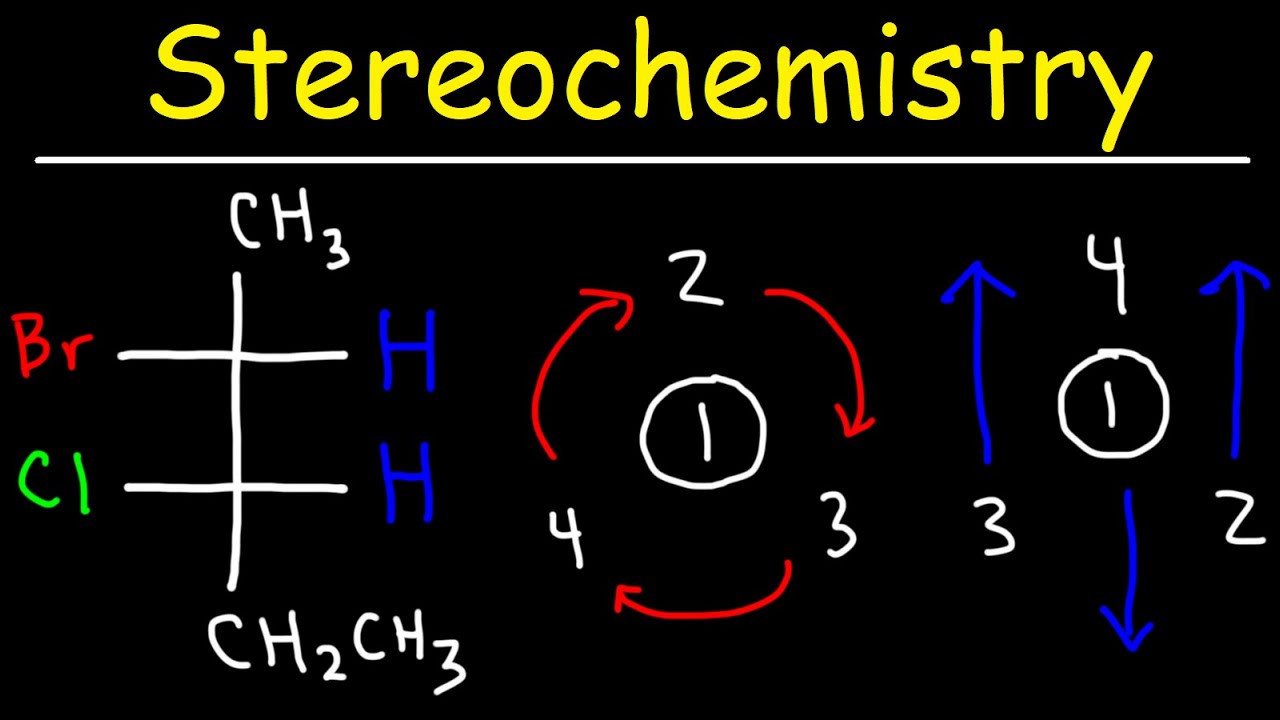
- 🔄 For Fischer projections, if the lowest priority group (usually hydrogen) is on a vertical bond, it is considered to be away from the viewer, simplifying the R/S assignment.
- 🔄 Conversely, if the lowest priority group is on a horizontal bond, it is towards the viewer, and the R/S assignment must be inverted after tracing the priority order.
- 🔄 A clockwise sequence from 1 to 2 to 3 in the priority order results in the R configuration, while a counterclockwise sequence results in the S configuration.
- 🔄 The tutorial emphasizes the need to remember that swapping two groups on a stereocenter inverts the stereochemistry, which is crucial for correct R/S assignment.
- 📖 The script suggests watching a previous tutorial on the CIP convention for a deeper understanding of the process before applying it to Fischer projections.
Q & A
What is the purpose of discussing Fischer projections in the context of stereochemistry?
-Fischer projections are discussed to help students understand an alternative notation system for representing stereochemistry, especially for those learning it in other introductory organic chemistry courses.
Why might some introductory organic chemistry courses prefer using Fischer projections over other methods?
-Some courses may prefer Fischer projections because it is a traditional way to introduce stereochemistry, particularly with simple sugars like monosaccharides.
How does the Fischer projection notation differ from the regular line notation in terms of representing the spatial arrangement of atoms?
-In Fischer projections, all bonds are represented flat without wedges and dashes, with the understanding that horizontal bonds imply wedge bonds and vertical bonds imply dash bonds, unlike regular line notation which uses three-dimensional representations.
What is the significance of the 'bowtie' shape in remembering the stereochemistry implied by Fischer projections?
-The 'bowtie' shape helps visualize that each carbon atom has a tetrahedral geometry, with one bond projecting outwards and three inwards, which is a key aspect of understanding the spatial arrangement in Fischer projections.
How can one visualize a Fischer projection to better understand the actual three-dimensional geometry of the molecule?
-One can visualize a Fischer projection by imagining the molecule in an imaginary plane, with certain atoms or groups coming towards the viewer and others going away, to help understand the tetrahedral arrangement of sp3 hybridized carbons.
What is the correct way to assign R/S configuration to a stereocenter in a Fischer projection when the lowest priority group is on a vertical bond?
-When the lowest priority group is on a vertical bond, which is implied to be a dash bond, it is already facing away from the viewer. In this case, you can assign R/S by tracing from the highest to the next highest priority groups in a clockwise or counterclockwise direction.
How should one approach assigning R/S configuration to a stereocenter in a Fischer projection when the lowest priority group is on a horizontal bond?
-If the lowest priority group is on a horizontal bond, which is implied to be a wedge bond, you should first trace from the highest to the next highest priority groups as if the lowest priority group is away from you, and then invert the result to account for the actual orientation.
What is the role of the Cahn-Ingold-Prelog (CIP) priority rules in assigning R/S configuration in Fischer projections?
-The CIP priority rules are essential for assigning R/S configuration as they dictate the priority of substituents based on atomic number, which helps determine the correct sequence for tracing around the stereocenter.
Can you provide an example of how to assign R/S configuration to a Fischer projection with a bromine, chlorine, fluorine, and hydrogen attached to a carbon?
-In this example, bromine would have the highest priority (1), followed by chlorine (2), fluorine (3), and hydrogen (4). If hydrogen is on a vertical bond, you would trace from bromine to chlorine to fluorine, and if the sequence is clockwise, the configuration would be R.
What is the common mistake to avoid when assigning R/S configuration to a Fischer projection with the lowest priority group on a horizontal bond?
-The common mistake is to directly trace from the highest to the next highest priority groups without considering the inversion of stereochemistry that occurs when the lowest priority group is actually towards the viewer. The correct approach is to trace as if the lowest group is away and then invert the result.
Outlines
🔍 Understanding Fischer Projections in Stereochemistry
This paragraph delves into the concept of Fischer projections, a method used to represent stereochemistry, particularly in the context of organic chemistry. The speaker, Professor Dave, explains that while he prefers line notation, Fischer projections are commonly taught in introductory organic chemistry courses. The paragraph clarifies that in Fischer projections, horizontal lines represent wedge bonds, and vertical lines represent dash bonds, implying a tetrahedral arrangement around carbon atoms. The speaker also discusses the challenges of visualizing the three-dimensional structure from a two-dimensional representation and suggests imagining the molecule from an 'imagined urine plane' perspective. The paragraph concludes with the importance of understanding this notation for various applications, including the assignment of R/S configurations using the Cahn-Ingold-Prelog convention, which is assumed to be known from previous tutorials.
📚 Assigning R/S Configurations Using Fischer Projections
In this paragraph, the focus shifts to the practical application of assigning R/S configurations to stereocenters using Fischer projections. The speaker outlines the process, emphasizing the importance of the Cahn-Ingold-Prelog priority rules. The paragraph explains that if the lowest priority group (hydrogen in this case) is on a vertical bond, it is considered to be away from the viewer, simplifying the assignment of R/S configurations. However, if the lowest priority group is on a horizontal bond, the process requires an inversion of the stereochemistry after tracing the priority order from 1 to 2 to 3. The speaker provides examples to illustrate the correct approach, highlighting the common mistake of not accounting for the inversion when the lowest priority group is facing the viewer. The paragraph aims to ensure that students can accurately assign R/S configurations to molecules represented in Fischer projection notation.
Mindmap
Keywords
💡Fischer Projections
💡Stereochemistry
💡Chirality
💡Cahn-Ingold-Prelog Convention
💡Wedge and Dash Bonds
💡Tetrahedral Centers
💡Methyl Groups
💡Hydroxyl Groups
💡Atomic Number
💡R/S Configuration
Highlights
Fischer projections are a common notation in organic chemistry for representing molecules, especially used for linear sugars and stereochemistry.
In Fischer projections, horizontal bonds are implied to be wedge bonds (coming out of the plane), and vertical bonds are implied to be dash bonds (going into the plane).
Each vertex and end point in a Fischer projection is implied to be a carbon atom.
Understanding the geometry: In Fischer projections, a horizontal bond implies a wedge bond, and a vertical bond implies a dash bond.
A visual trick to remember the bond directions in Fischer projections is to imagine a bowtie at each carbon.
For longer molecules, Fischer projections may appear illogical, but can be visualized as a horseshoe shape or a rollercoaster.
When assigning R and S configuration using Fischer projections, the lowest priority group must be facing away from the observer.
In Fischer projections, if the lowest priority group is on a vertical bond, it is already facing away (dash bond).
If the lowest priority group is on a horizontal bond (wedge bond), the configuration must be assessed and then inverted.
Assign priorities based on atomic number to determine the R and S configuration.
Trace from priority 1 to 2 to 3: if clockwise, it's R; if counterclockwise, it's S.
Inverting stereochemistry: Swapping two groups on a stereocenter inverts the configuration.
Practical application: This method helps in assigning stereochemistry to molecules drawn in Fischer projection form.
Key takeaway: Vertical bonds imply dash (away), horizontal bonds imply wedge (towards), and correct stereochemistry assignment requires understanding these conventions.
For consistent results, always ensure the lowest priority group is correctly positioned or account for its position through inversion.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: