Cahn-Ingold-Prelog Convention (Determining R/S)
TLDRThis script explains the process of assigning absolute configuration to chiral centers using the Cahn-Ingold-Prelog (CIP) convention. It details identifying chiral centers, assigning priorities to substituents based on atomic mass, and determining R or S stereochemistry by visualizing the molecule from a privileged perspective. The tutorial also addresses strategies for assigning configurations when the lowest priority group is not oriented away from the viewer, including mental rotation and swapping groups to simplify the assessment, with a reminder to invert the result if groups are switched.
Takeaways
- 🧬 Chirality in Molecules: A molecule with one chiral center has two enantiomeric forms, requiring a method to assign absolute configuration.
- 📜 Cahn-Ingold-Prelog Convention: This is the method used to differentiate between enantiomers and assign absolute configuration to chiral centers.
- 🔍 Identifying Chiral Centers: A chiral center is identified by a carbon atom connected to four different groups.
- 🔢 Atomic Mass Prioritization: To assign priorities to the groups around a chiral center, one should consider the atomic mass of the atoms, starting with the heaviest.
- 🔄 Equivalency and Chain Extension: In cases of equivalency between carbon atoms, extend the chain until a point of difference is found to determine priority.
- 👀 Viewing Perspective: To assign absolute configuration, the molecule should be viewed such that the lowest priority group is away from the viewer.
- 🔄 Clockwise vs. Counterclockwise: The direction of the circle formed by the groups from 1 to 2 to 3 determines whether the stereocenter is R (clockwise) or S (counterclockwise).
- 🔄 Stereochemistry Inversion: Swapping any two groups on a chiral center inverts its stereochemistry, which must be considered when assigning absolute configuration.
- 📐 Spatial Reasoning: Assigning absolute configuration may require mental rotation or repositioning of the molecule to achieve the correct viewing perspective.
- 🔄 Arbitrary Group Swapping: To simplify the process, one can swap two groups on the chiral center, but must then invert the observed configuration to get the original molecule's absolute configuration.
- 📚 Continuous Learning: The tutorial encourages viewers to subscribe for more educational content and to reach out with questions for further clarification.
Q & A
What is a chiral center in chemistry?
-A chiral center is a point in a molecule where the arrangement of atoms is non-superimposable on its mirror image, typically involving a carbon atom bonded to four different groups.
Why is it important to differentiate between enantiomers?
-Differentiating between enantiomers is crucial because they can have different chemical properties, reactions, and biological activities, which is particularly important in pharmaceuticals and organic chemistry.
What is the Cahn-Ingold-Prelog (CIP) convention?
-The Cahn-Ingold-Prelog convention is a set of rules used to assign the absolute configuration of chiral centers, determining whether a molecule is R (rectus) or S (sinister) based on the priority of the attached groups.
How is the priority of groups at a chiral center determined?
-The priority is determined by the atomic mass of the atoms directly attached to the chiral center, with the heaviest atom having the highest priority and the lightest having the lowest.
What is the significance of the implied hydrogen in the chiral center?
-The implied hydrogen is considered as a group with the lowest atomic mass, which is used for determining the priority of the groups attached to the chiral center.
How do you handle equivalency of groups when assigning priorities?
-In case of equivalency, one must extend the analysis down the chain until a point of difference is reached, comparing the atomic masses of the additional atoms to determine the priority.
What is the privileged perspective for assigning absolute configuration?
-The privileged perspective is when the lowest priority group is positioned away from the observer, allowing for a direct assignment of the absolute configuration without needing to rotate or redraw the molecule.
How do you determine if a chiral center is R or S?
-You determine the absolute configuration by visualizing the path from the highest priority group to the next highest, and so on, until you reach the lowest priority group. If the path is clockwise, it is R; if counterclockwise, it is S.
What if the lowest priority group is not away from you in the molecule's representation?
-If the lowest priority group is not away, you can either mentally rotate the molecule, change your perspective, or use a 'cheating' method by swapping two groups to make the lowest priority group away, but then you must invert the configuration you observe.
What is the consequence of swapping two groups on a chiral center?
-Swapping two groups on a chiral center will invert the stereochemistry of that center. Therefore, after swapping, the observed configuration must be inverted to reflect the original molecule's absolute configuration.
Why might one need to redraw or rotate a molecule when assigning absolute configuration?
-Redraw or rotate a molecule when assigning absolute configuration to ensure that the lowest priority group is away from the observer, which simplifies the process of determining whether the configuration is R or S.
Outlines
🧪 Assigning Absolute Configuration to Chiral Centers
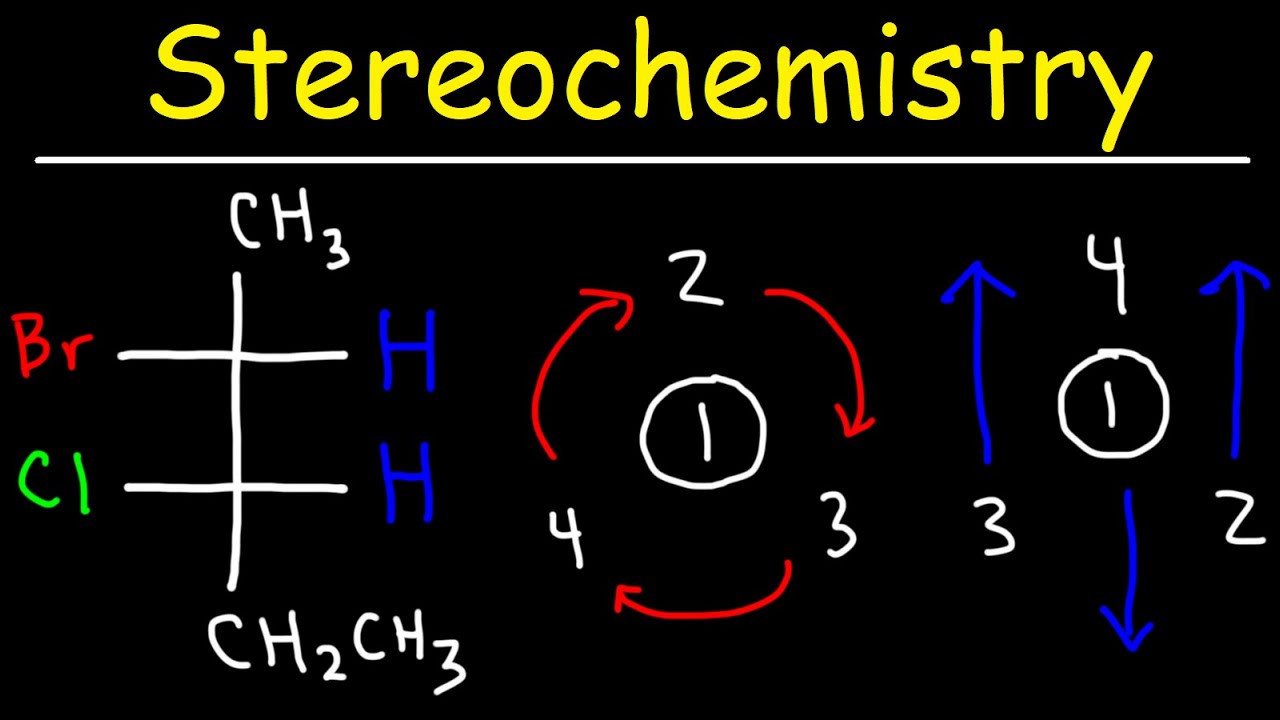
This paragraph explains the concept of absolute configuration for chiral centers in molecules. It introduces the Cahn-Ingold-Prelog (CIP) priority rules for assigning the configuration. The process involves identifying the chiral center, assigning priorities to the four different groups attached to it based on atomic mass, and then determining the absolute configuration (R or S) by visualizing the molecule such that the lowest priority group is away from the viewer. The example given is for (R)-2-butanol, where the molecule is already oriented correctly for easy assignment.
🔍 Techniques for Assigning Absolute Configuration in Various Orientations
This paragraph delves into methods for assigning absolute configuration when the molecule's orientation is not initially favorable. It suggests mental rotation or spatial reorientation as one approach for those proficient in spatial reasoning. For others, it proposes a 'cheating' method where two groups are swapped to facilitate easier configuration assignment, but cautions that the determined configuration must then be inverted to reflect the original molecule's stereochemistry. Examples are provided to illustrate the process, emphasizing the importance of considering the orientation of substituents in space or on paper.
📚 Strategies for Determining Stereochemistry of Chiral Centers
The final paragraph summarizes the strategies for determining the stereochemistry of chiral centers, especially when the lowest priority group is not oriented away from the viewer. It reiterates the two main approaches: mentally changing the viewer's perspective to align with the molecule's spatial arrangement or swapping any two groups on the chiral center and inverting the observed configuration. The paragraph concludes with an invitation for viewers to subscribe for more tutorials and to reach out with questions, reinforcing the educational nature of the content.
Mindmap
Keywords
💡Chiral Center
💡Enantiomers
💡Cahn-Ingold-Prelog (CIP) Convention
💡Atomic Mass
💡Priority
💡Absolute Configuration
💡Stereocenter
💡R Configuration
💡S Configuration
💡Spatial Orientation
💡Inversion of Chiral Center
Highlights
Introduction to the concept of chiral centers and the need for a method to differentiate between enantiomers.
Explanation of the Cahn-Ingold-Prelog convention for assigning absolute configuration to chiral centers.
Identification of a chiral center in a molecule based on its connection to four different groups.
The process of assigning priority to the groups attached to a chiral center based on atomic mass.
Differentiating between equivalent carbon atoms by extending the chain until a point of difference is found.
The importance of assigning priority one atom at a time, rather than considering entire groups.
Technique for determining the absolute configuration by placing the molecule so the lowest priority group is away from the observer.
Method for assigning absolute configuration using a clockwise or counterclockwise circle from group 1 to 2 to 3.
Identification of (R)-2-butanol using the Cahn-Ingold-Prelog convention.
Strategies for assigning absolute configuration when the lowest priority group is not initially away from the observer.
Mental rotation or spatial reorientation techniques for assigning absolute configuration.
The 'cheating method' of swapping groups on a chiral center to facilitate easier configuration assignment.
The necessity of inverting the stereochemistry result when using the swapping method.
Handling chiral centers with the lowest priority group in the plane of the board and the use of spatial reasoning.
The rule of inverting the stereochemistry when any two groups on a chiral center are swapped.
Final summary of the process for assigning absolute configuration to chiral centers in various orientations.
Encouragement for viewers to subscribe for more tutorials and to reach out with questions.
Transcripts
Browse More Related Video

5.2 How to Assign R and S | Absolute Configuration | Organic Chemistry
Stereochemistry - R S Configuration & Fischer Projections

More Examples Using the Cahn-Ingold-Prelog Convention

Enantiomers

E/Z Absolute Configuration of Alkenes

5.3 Molecules with Multiple Chiral Centers | Enantiomers, Diastereomers, and Meso Compounds | OChem
5.0 / 5 (0 votes)
Thanks for rating: