5.2 How to Assign R and S | Absolute Configuration | Organic Chemistry
TLDRThe video script is a detailed lesson on absolute configurations of chiral centers in chemistry, as part of a broader series on isomers and stereochemistry. The presenter, Chad, explains the Cahn-Ingold-Prelog priority rules system used to assign the R or S designation to chiral centers. He clarifies that these designations help distinguish between two forms of a chiral center that are mirror images of each other. Chad provides a step-by-step guide on how to assign priorities to the four different groups attached to a chiral center based on atomic number and, in case of a tie, by examining the additional atoms bonded to the tied carbons. He then demonstrates how to visualize the molecule to determine if the lowest priority group is in a dashed (away from the observer) or wedged (towards the observer) position to make a right- or left-handed turn, respectively. The lesson includes examples of how to assign R or S configurations to various molecules and how to name molecules with chiral centers, emphasizing the importance of distinguishing between different enantiomers. The summary aims to make the complex topic of stereochemistry more understandable and potentially enjoyable for viewers.
Takeaways
- 🔍 **Absolute Configurations:** The Cahn-Ingold-Prelog (CIP) system is used to assign the R or S designation to chiral centers to distinguish between their two different forms (enantiomers).
- ⚙️ **Chiral Center Recognition:** A chiral center is an atom, usually carbon, bonded to four different groups, which can exist in two mirror-image forms.
- 🔢 **Priority Assignment:** In the CIP system, the four groups attached to a chiral center are assigned priorities from 1 to 4 based on atomic number, with 1 being the highest priority.
- 🔄 **Rule of Four:** When two carbon atoms are tied in priority, the atom bonded to them is examined, and the one with the higher atomic number takes precedence.
- ↔️ **Clockwise/Counterclockwise:** The R (rectus) designation is given for a right-handed turn, and the S (sinister) for a left-handed turn when viewing the molecule with the lowest priority group in a dashed (or going away) position.
- 🖐 **Viewpoint Consideration:** The perspective from which the molecule is viewed is crucial; if the lowest priority group is not in a dashed position, the molecule may need to be mentally flipped or the viewpoint changed.
- 🔀 **Group Interchange:** Exchanging any two groups on a chiral center inverts the stereochemistry, switching R and S configurations.
- 📚 **Naming Chiral Molecules:** Molecules with chiral centers are named by adding the R or S designation in parentheses before the name of the molecule, with the location number when there are multiple chiral centers.
- ⛓ **Double Bond Consideration:** In the priority assignment, a double bond counts as two separate bonds to the same atom when comparing atomic numbers.
- 🔬 **Complex Molecule Analysis:** For molecules with multiple chiral centers or complex structures, the priority of each center is determined independently, and the molecule is named accordingly.
- 🧠 **Mental Rotation vs. Group Swap:** For students struggling with three-dimensionality, swapping groups instead of rotating bonds can simplify the assignment of R or S configurations.
- 📈 **Practice and Understanding:** Proficiency in assigning R and S configurations comes with practice and understanding the logic behind the CIP system's rules.
Q & A
What are absolute configurations?
-Absolute configurations are designations given to a chiral center that help distinguish between two different forms of a chiral center that are mirror images of each other. They are designated as 'R' or 'S' according to the Cahn-Ingold-Prelog priority rules system.
What is a chiral center?
-A chiral center is an atom, typically a carbon, that is bonded to four different groups and can exist in two different forms that are mirror images of each other, known as enantiomers.
How does the Cahn-Ingold-Prelog system assign priorities to the groups attached to a chiral center?
-The Cahn-Ingold-Prelog system assigns priorities based on atomic number. The group with the highest atomic number gets the highest priority (1), and the lowest, typically hydrogen, gets the lowest priority (4). In case of a tie, the system looks at the atoms bonded to the carbons in question and compares them in descending order of atomic number.
What does the term 'R' stand for and what does it represent in stereochemistry?
-'R' stands for 'rectus', which is Latin for 'right'. In stereochemistry, it represents a right-handed turn when the lowest priority group (usually hydrogen) is pointing away from the observer in a dashed bond.
What does the term 'S' stand for and what does it represent in stereochemistry?
-'S' stands for 'sinister', which is Latin for 'left'. In stereochemistry, it represents a left-handed turn when the lowest priority group is pointing away from the observer in a dashed bond.
How do you determine the configuration (R or S) if the lowest priority group is not in a dashed position?
-If the lowest priority group is not in a dashed position but is a wedge bond, you would look at the molecule from the opposite perspective (as if you were looking from the other side), and the handedness of the turn would be opposite. If the lowest priority group is in the plane of the drawing, one approach is to swap two groups on the chiral center to assign the configuration more easily.
How does the presence of a double bond affect the priority assignment in the Cahn-Ingold-Prelog system?
-When there is a double bond, each bond is counted separately. For example, a double bond to an oxygen would be counted as two separate bonds to oxygen in the priority assignment.
What is the correct way to name a molecule with multiple chiral centers?
-For molecules with multiple chiral centers, you should include the 'R' or 'S' designation in parentheses at the beginning of the name, followed by the carbon location number where the chiral center is located. If there are two chiral centers, you would list them in numerical order, separated by a comma (e.g., 2R,3S-2-chloro-3-methylhexane).
What is the significance of distinguishing between the 'R' and 'S' configurations of a chiral molecule?
-The 'R' and 'S' configurations are significant because they represent different enantiomers of a chiral molecule, which can have different chemical properties, reactivity, and biological activity. This distinction is crucial in fields such as drug development and organic chemistry.
How can students struggle with the three-dimensionality of assigning R or S configurations?
-Students often struggle with the three-dimensionality because they may not visualize the molecule correctly from the perspective required by the Cahn-Ingold-Prelog system. They might also have difficulty in determining the correct priority of groups when there are ties or when double bonds are present.
What is one strategy to simplify the assignment of R or S configurations for students new to organic chemistry?
-One strategy is to swap two groups on the chiral center to create a new configuration that is easier to assign as R or S. This method helps avoid the complexity of rotating bonds or struggling with three-dimensional visualization, especially for students new to the subject.
Outlines
🧪 Understanding Absolute Configurations and Chiral Centers
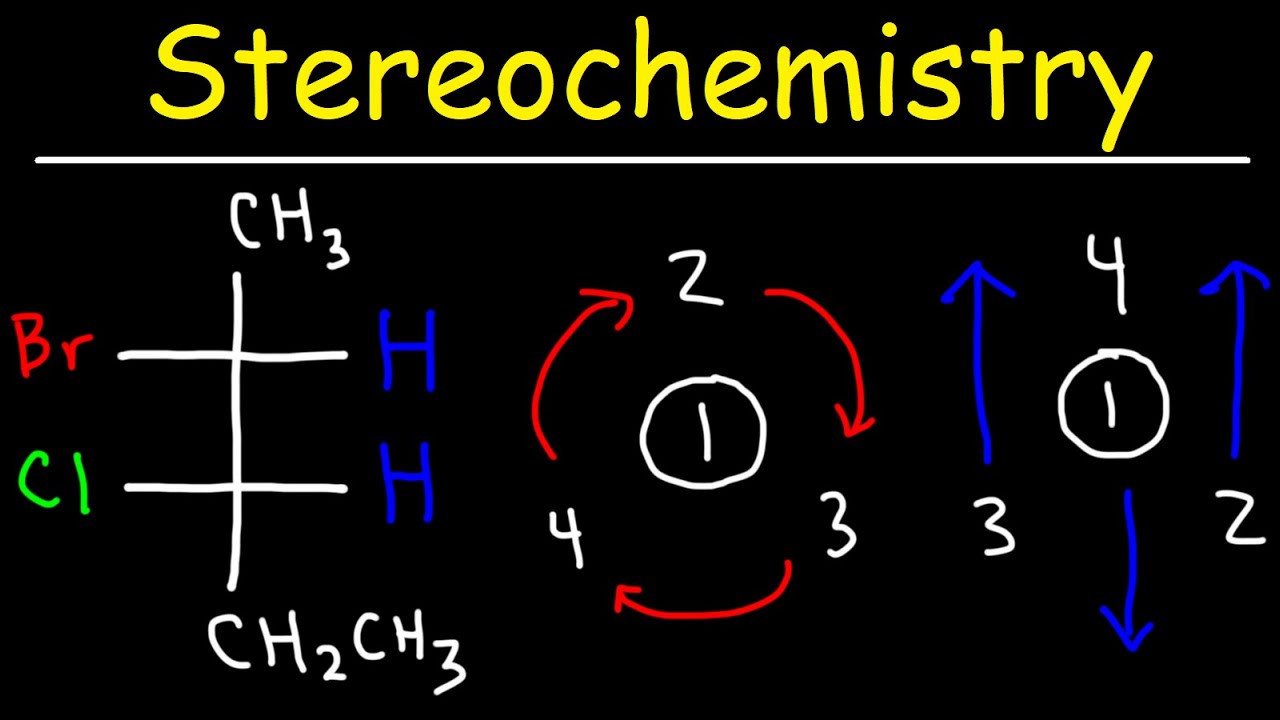
This paragraph introduces the concept of absolute configurations as they relate to chiral centers in chemistry. A chiral center is a carbon atom bonded to four different groups and exists in two forms that are mirror images of each other. The Cahn-Ingold-Prelog (CIP) system is explained as a set of rules that designate these forms as either R or S. The process involves assigning priorities to the four groups attached to the chiral center based on atomic number and comparing them to distinguish between the R and S configurations. The importance of perspective when determining the configuration is emphasized, as the same molecule can appear as a right-handed or left-handed turn depending on the viewer's angle.
🔍 Assigning R and S Configurations in Complex Molecules
The second paragraph delves into the complexities of assigning R and S configurations when the lowest priority group is not in a dashed position. It explains how to approach molecules where the lowest priority group is a wedge bond by considering the perspective from the opposite side. The paragraph also addresses the most challenging scenario where the lowest priority group is in the plane of the paper, and a common student approach to rotate bonds is critiqued. An alternative method is suggested, which involves swapping two groups on the chiral center to assign priorities more easily. This method is recommended for students who have prior experience with organic chemistry.
📚 Navigating Tricky Examples and Prioritizing Groups
This paragraph presents more complex examples of assigning R and S configurations, highlighting common pitfalls and how professors may present more difficult examples on exams than in class. The process of assigning priorities to groups attached to a chiral center is detailed, with special attention given to instances where the groups are tied. The paragraph explains how to break ties by considering additional atoms bonded to the carbons and the importance of the double bond counting as separate bonds when comparing priorities. The CIP system's rule of looking for the first point of difference is emphasized for making these determinations.
🔬 Resolving Ties in Chiral Center Prioritization
The fourth paragraph continues the discussion on resolving ties when assigning priorities to chiral centers. It provides a step-by-step method for determining the priority of carbons bonded to hydrogens and other carbons. The concept of counting pi bonds back to the previous atom is introduced when a carbon is bonded to fewer than three additional atoms. The paragraph concludes with a reminder that once a tie is resolved, the process of assigning a right-handed or left-handed turn to determine R or S configuration can be completed, taking into account the perspective from which the molecule is viewed.
📝 Naming Molecules with Chiral Centers
This paragraph focuses on the naming of molecules that have chiral centers. It explains that the molecule's name must be adjusted to reflect the R or S configuration at each chiral center. The process of naming involves first identifying the longest continuous carbon chain and then adding prefixes for substituents like chlorine. The paragraph emphasizes the need to include the R or S designation in the name when there are chiral centers present. It also covers how to name molecules with multiple chiral centers by incorporating the configuration and position of each center into the name.
🌟 Engaging with the Content and Seeking Further Resources
The final paragraph encourages viewers to engage with the content by liking and sharing the video if they found it helpful. It also invites viewers to ask questions in the comment section, where the presenter is active. Additionally, it promotes the presenter's premium course on chatsprep.com for further practice problems and study guides related to the topic of stereochemistry and chiral centers.
Mindmap
Keywords
💡Absolute Configurations
💡Chiral Center
💡Cahn-Ingold-Prelog (CIP) System
💡Enantiomers
💡Stereochemistry
💡Atomic Number
💡Wedge and Dash Bonds
💡R and S Designations
💡Organic Chemistry
💡Stereoisomers
💡Priority Assignment
Highlights
Lesson focuses on absolute configurations and the Cahn-Ingold-Prelog system for designating chiral centers as R or S.
Chiral centers exist as two different forms that are mirror images of each other.
The Cahn-Ingold-Prelog system prioritizes the four different groups attached to a chiral center based on atomic number.
If there's a tie in atomic numbers, deeper inspection of the atoms bonded to the carbons is required to assign priority.
The R and S designation comes from the direction of the turn when viewing the molecule - R for right-handed and S for left-handed.
The perspective is crucial as the molecule may appear to turn in one direction from one side and the opposite from the other.
When assigning configurations, the lowest priority group must be in the dashed position going away from the viewer for a correct R or S assignment.
If the lowest priority group is not in the dashed position, the molecule may need to be mentally flipped or the perspective changed to assign correctly.
For more complex examples, students are advised to swap groups on the chiral center to simplify the assignment of R or S.
Swapping two groups on a chiral center inverts the stereochemistry, helping in assigning R or S configurations.
The video provides a method to handle cases where the lowest priority group is in the plane of the paper, a common point of difficulty for students.
When naming molecules with chiral centers, the R or S configuration must be included in the name to distinguish between enantiomers.
For molecules with multiple chiral centers, the R or S designation is combined with the carbon position for precise naming.
The video emphasizes the importance of practice in assigning R and S configurations and naming chiral molecules.
Chad provides a premium course for additional practice problems and study guides related to organic chemistry and stereochemistry.
The lesson is part of a weekly organic chemistry playlist released throughout the 2020-21 school year.
Subscribing to Chad's Prep channel and enabling notifications will ensure viewers are updated with each new lesson release.
The method for assigning R and S configurations involves comparing atomic numbers and visualizing the molecule's three-dimensional structure.
Transcripts
Browse More Related Video

Cahn-Ingold-Prelog Convention (Determining R/S)
Stereochemistry - R S Configuration & Fischer Projections

5.3 Molecules with Multiple Chiral Centers | Enantiomers, Diastereomers, and Meso Compounds | OChem

Stereochemistry: Meso Compounds, Diastereomers

5.1 Overview of Isomers | Constitutional Isomers and Stereoisomers | Organic Chemistry

5.4 Fischer Projections | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: