What is entropy? - Jeff Phillips
TLDRThe video script delves into the concept of entropy, a fundamental principle in chemistry and physics that explains the directionality of physical processes. It clarifies misconceptions about entropy as a measure of disorder and instead frames it as a measure of energy spread, illustrated through the probability of energy distribution in atomic bonds. The script uses a dynamic system model to demonstrate how energy naturally disperses, leading to spontaneous processes like cooling down of hot objects. It emphasizes that higher entropy states are statistically more probable, illustrating why physical changes like ice melting occur and why they are irreversible in everyday life.
Takeaways
- 🔍 Entropy is a fundamental concept in chemistry and physics that explains the directionality of physical processes.
- 🧊 Entropy is often misunderstood as a measure of disorder, but it's more accurately described as a measure of energy distribution.
- 📏 Entropy is better understood through the lens of probability and the concept of microstates, which represent different ways energy can be distributed.
- 🔥 The more energy a substance has, the more microstates it can have, leading to a higher entropy.
- 🔄 Entropy is a measure of the likelihood of different energy configurations, with configurations that spread energy out having higher entropy.
- ⚖️ Entropy increases as energy becomes more dispersed, and decreases when energy is concentrated in fewer states.
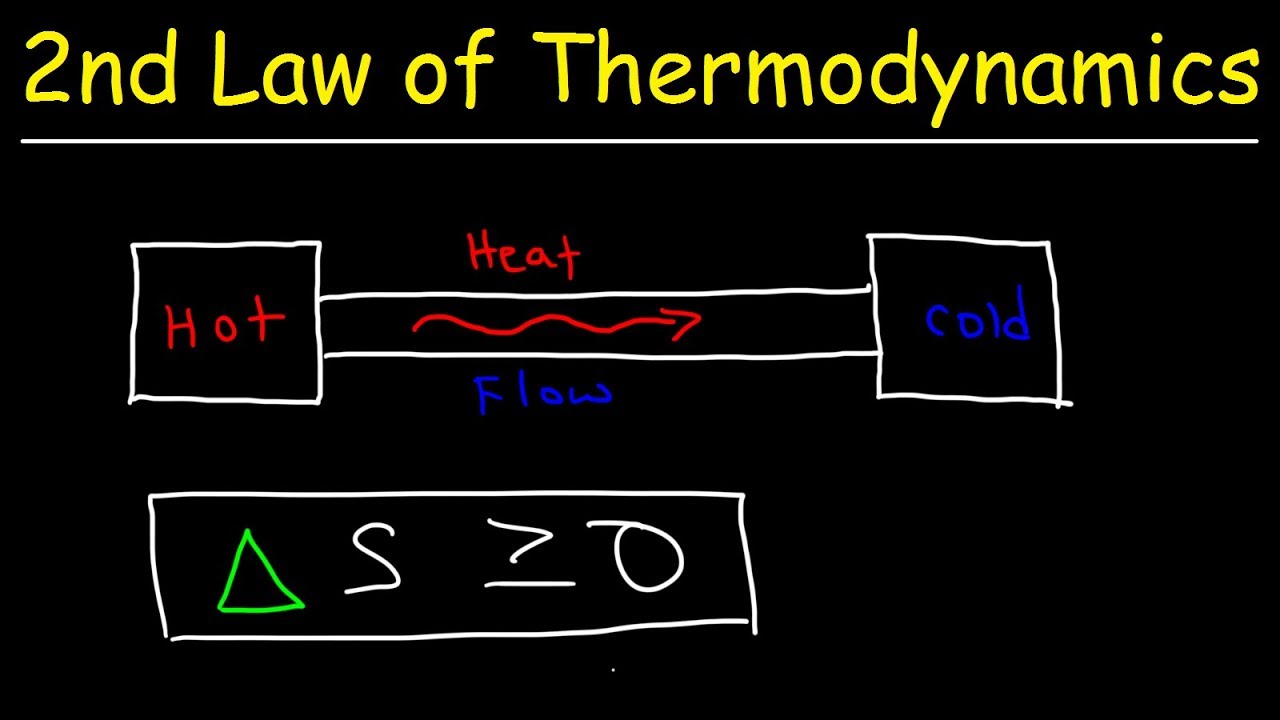
- 🌡️ Spontaneous processes, such as heat transfer, can be explained by the tendency of energy to spread out to configurations of higher entropy.
- 🔄 The movement of energy between bonds in a dynamic system can change the energy configuration, often leading to an increase in entropy.
- 🔮 In larger systems, the probability of energy configurations with higher entropy occurring is so much greater that they are effectively inevitable.
- 🏔️ The melting of ice, spreading of cream in coffee, and deflation of tires are examples of processes moving towards higher entropy states.
- ⏳ Entropy has been called 'time's arrow' because it reflects the statistical likelihood of energy spreading out over time in any system that allows it.
Q & A
What is the concept that helps explain why physical processes go one way and not the other?
-The concept is entropy, which is crucial to chemistry and physics and helps explain phenomena such as why ice melts, cream spreads in coffee, and air leaks out of a punctured tire.
Why is entropy often considered difficult to understand?
-Entropy is difficult to understand because it is often described as a measurement of disorder, which can be misleading, as it is more accurately a measure of the spread of energy.
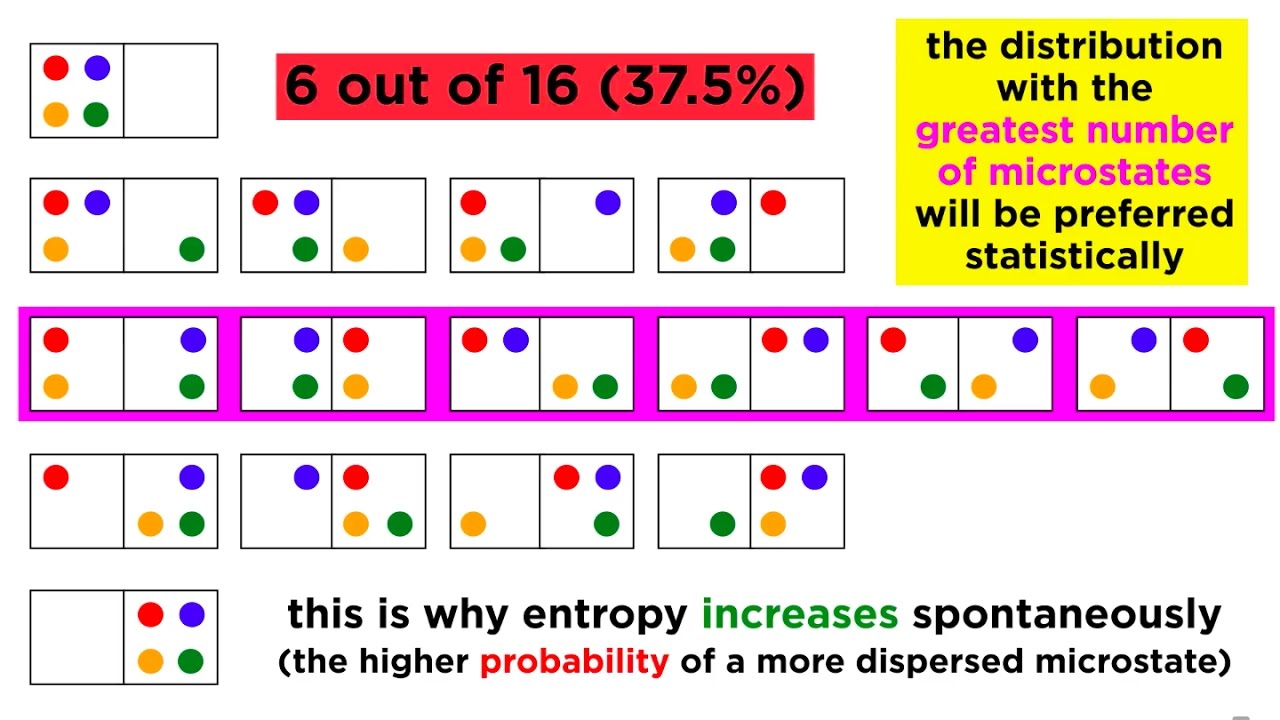
What is a microstate in the context of entropy?
-A microstate is one of the numerous ways that energy can be distributed among particles or bonds in a system, with each microstate representing a possible configuration of energy.
How does the number of microstates relate to the concept of entropy?
-The number of microstates is directly related to entropy. A higher number of microstates indicates a higher probability of a particular energy configuration occurring, which corresponds to higher entropy.
What does it mean for a system to have low entropy?
-Low entropy means that the energy in the system is concentrated or less spread out among the particles or bonds.
What does it mean for a system to have high entropy?
-High entropy means that the energy in the system is spread out or dispersed among the particles or bonds, indicating a greater number of microstates and a higher probability of occurrence.
How does entropy relate to the spontaneous cooling of a hot object?
-Entropy is useful for explaining spontaneous processes like the cooling of a hot object because the energy tends to spread out to achieve a higher entropy state, which is statistically more likely.
Why does the energy configuration with the energy most spread out between solids have the highest entropy?
-The energy configuration with the energy most spread out has the highest entropy because it has the greatest number of microstates, indicating a higher probability of occurrence.
What is the significance of the size of the system in the context of entropy and spontaneous processes?
-The size of the system is significant because larger systems have an astronomically higher number of microstates, making it statistically unlikely for energy to become concentrated again once it has dispersed.
Why is entropy sometimes referred to as 'time's arrow'?
-Entropy is referred to as 'time's arrow' because it indicates the direction in which energy will naturally spread out over time, from a state of lower entropy to a state of higher entropy.
How does the script explain the melting of ice, spreading of cream in coffee, and deflation of tires in terms of entropy?
-The script explains these phenomena as examples of spontaneous processes that occur because the resulting states have more dispersed energy and higher entropy, which is statistically more likely.
Outlines
🔍 Entropy: The Measure of Energy Spread
This paragraph introduces entropy as a fundamental concept in chemistry and physics that explains the directionality of physical processes. It challenges the common misconception of entropy as disorder, instead suggesting that it should be viewed through the lens of probability and energy distribution. The concept of microstates is introduced to illustrate how entropy can be understood as a measure of the spread of energy, with higher entropy indicating a more dispersed energy configuration and a higher probability of occurrence. The paragraph also touches on the dynamic nature of energy, which naturally moves and changes configurations, leading to a tendency for energy to spread out, as seen in everyday phenomena like melting ice or cooling hot objects.
Mindmap
Keywords
💡Entropy
💡Disorder
💡Microstate
💡Quanta
💡Probability
💡Energy Spread
💡Spontaneous Processes
💡Dynamic System
💡Statistical Likelihood
💡Time's Arrow
💡Concentration vs. Dispersion
Highlights
Entropy is a crucial concept in chemistry and physics that explains the directionality of physical processes.
Entropy is often misunderstood as a measurement of disorder, but it is more accurately described through the lens of probability.
The concept of microstates illustrates the various ways energy can be distributed within a system.
A higher number of microstates corresponds to a higher probability of a particular energy configuration occurring.
Entropy is a direct measure of the probability of energy configurations, indicating how spread out the energy is within a system.
Low entropy signifies concentrated energy, while high entropy indicates energy dispersion.
Energy tends to move and spread out due to the higher probability of dispersed energy configurations.
The example of two solids with different energy distributions demonstrates how entropy can explain spontaneous processes.
In dynamic systems, energy continuously moves between neighboring bonds, leading to changes in energy configurations.
The probability of energy being maximally spread out is higher, which is why entropy is considered as time's arrow.
Real-world objects have such a vast number of particles that the chance of energy becoming concentrated is negligible.
The melting of ice, mixing of cream in coffee, and deflation of tires are examples of processes driven by higher entropy states.
There is no external force pushing systems towards higher entropy; it is a natural statistical likelihood.
The concept of entropy helps explain why certain spontaneous processes occur and why they are unidirectional.
Understanding entropy provides insight into the natural tendency of energy to disperse and the statistical nature of physical processes.
The transcript emphasizes the importance of entropy in explaining why physical processes occur in one direction rather than another.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: