Organic Chemistry Exam 1 Review
TLDRThis video script offers an in-depth review of key topics for the first organic chemistry exam, focusing on alkane nomenclature, IUPAC rules, and functional group identification. It explains how to name alkanes, determine hybridization, bond angles, and molecular geometry. The script also covers concepts like bond strength, formal charge, resonance structures, and the major resonance contributors, providing a comprehensive guide for students preparing for organic chemistry exams.
Takeaways
- 🧪 Alkanes are hydrocarbons with single bonds between carbon atoms, and the script covers naming alkanes up to decane using IUPAC nomenclature.
- 🔡 IUPAC nomenclature prioritizes the longest carbon chain, the lowest numbering for substituents, and alphabetical order when substituents are the same distance from the end of the chain.
- 📐 The script explains how to determine the type of substituents such as methyl, ethyl, propyl, isopropyl, sec-butyl, and ter-butyl, which are essential for IUPAC naming.
- 🔑 The importance of numbering the carbon chain from the direction that gives the lowest possible numbers for substituents is highlighted, especially when dealing with complex molecules.
- 📚 Examples are given to illustrate the process of naming alkanes with multiple substituents, emphasizing the need for the correct order and numbering.
- 🔍 The script discusses functional groups such as alcohols, aldehydes, ketones, carboxylic acids, esters, amines, and alkyl halides, explaining their structures and how to name them.
- 📘 The difference between primary, secondary, and tertiary alcohols and amines is explained, along with the concept of hybridization and its effect on bond angles and molecular geometry.
- 📐 The strength and length of bonds are inversely related, with triple bonds being the shortest and strongest, and single bonds being the longest and weakest.
- ⚖️ Formal charge is calculated based on valence electrons, bonds, and lone pairs, which helps in understanding the stability of molecules and ions.
- 🔁 Resonance structures are introduced, showing how to draw them, identify the major contributor, and represent the resonance hybrid, which is the average structure that represents the true state of the molecule.
Q & A
What is the general formula for naming alkanes up to decadiene in the script?
-Alkanes are named based on the number of carbon atoms they contain, following the pattern CnH2n+2, where 'n' is the number of carbon atoms. The script lists alkanes from methane (CH4) to decadiene (C10H22), with names like ethane (C2H6), propane (C3H8), butane (C4H10), and so on, up to decadiene.
How is the IUPAC nomenclature for alkanes determined in the script?
-IUPAC nomenclature for alkanes involves identifying the longest continuous chain of carbon atoms and numbering the chain to give the substituents the lowest possible numbers. The substituents are then listed in front of the parent alkane name in alphabetical order, with their positions indicated by numbers.
What is a bond line structure as mentioned in the script?
-A bond line structure is a way of representing the structure of an alkane where the carbon atoms are implied by the endpoints of the lines, and the hydrogen atoms are shown as substituents on these lines. It is a simplified version of the full structural formula.
How does the script describe the naming of a compound with a seven-carbon chain and two methyl groups?
-The script describes the compound as '3,4-dimethylheptane'. This name is derived from the longest chain of seven carbons (heptane), with two methyl groups attached to the third and fourth carbon atoms.
What are the rules for naming substituents in IUPAC nomenclature according to the script?
-Substituents are named based on the number of carbon atoms in the substituent chain. A one-carbon substituent is 'methyl', two carbons is 'ethyl', three is 'propyl', and so on. When multiple substituents are present, they are listed in alphabetical order and numbered to give the lowest possible numbers.
What is the difference between a primary, secondary, and tertiary alcohol as explained in the script?
-A primary alcohol has the hydroxyl (-OH) group attached to a carbon that is connected to only one other carbon. A secondary alcohol has the -OH group on a carbon connected to two other carbons. A tertiary alcohol has the -OH group on a carbon connected to three other carbons.
How is the strength and length of a bond related in the script?
-The script explains that bond strength and length are inversely related. Triple bonds are the strongest but the shortest, followed by double and then single bonds. Conversely, single bonds are the weakest but the longest.
What is the formula for calculating the formal charge of an atom as mentioned in the script?
-The formal charge of an atom is calculated using the formula: Formal Charge = Valence Electrons - (Bonds + Lone Electrons). This formula accounts for the number of electrons an atom 'owns' in a molecule.
How does the script describe the concept of resonance structures?
-Resonance structures are different ways of drawing the same molecule that have the same connectivity of atoms but different electron arrangements. The script explains that resonance structures are drawn by moving electrons (not atoms), and the major resonance contributor is the structure that represents the molecule most accurately due to its greater stability.
What is the significance of hybridization in determining bond angles and molecular geometry as discussed in the script?
-Hybridization affects the shape of a molecule and the angles between bonds. SP hybridization results in a linear shape with a bond angle of 180 degrees, SP2 hybridization results in a trigonal planar shape with bond angles of approximately 120 degrees, and SP3 hybridization results in a tetrahedral shape with bond angles close to 109.5 degrees.
Outlines
📚 Organic Chemistry Exam Review
This paragraph introduces the video's purpose, which is to review topics commonly found in the first organic chemistry exam. It covers the basics of alkanes, including their molecular formulas and structural representations, and emphasizes the importance of knowing the names of alkanes up to decane. The paragraph also introduces IUPAC nomenclature, providing examples of how to name molecules with substituents, focusing on the correct numbering and placement of substituents to achieve the lowest possible numbers.
🔍 IUPAC Nomenclature and Substituent Prioritization
The paragraph delves deeper into IUPAC nomenclature, explaining the process of naming alkanes with multiple substituents. It illustrates how to determine the parent chain and how to number it to achieve the lowest sum of substituent numbers. The paragraph also discusses the importance of alphabetically ordering substituents and provides examples of naming complex molecules, including those with functional groups and complex substituents like sec-butyl and tert-butyl.
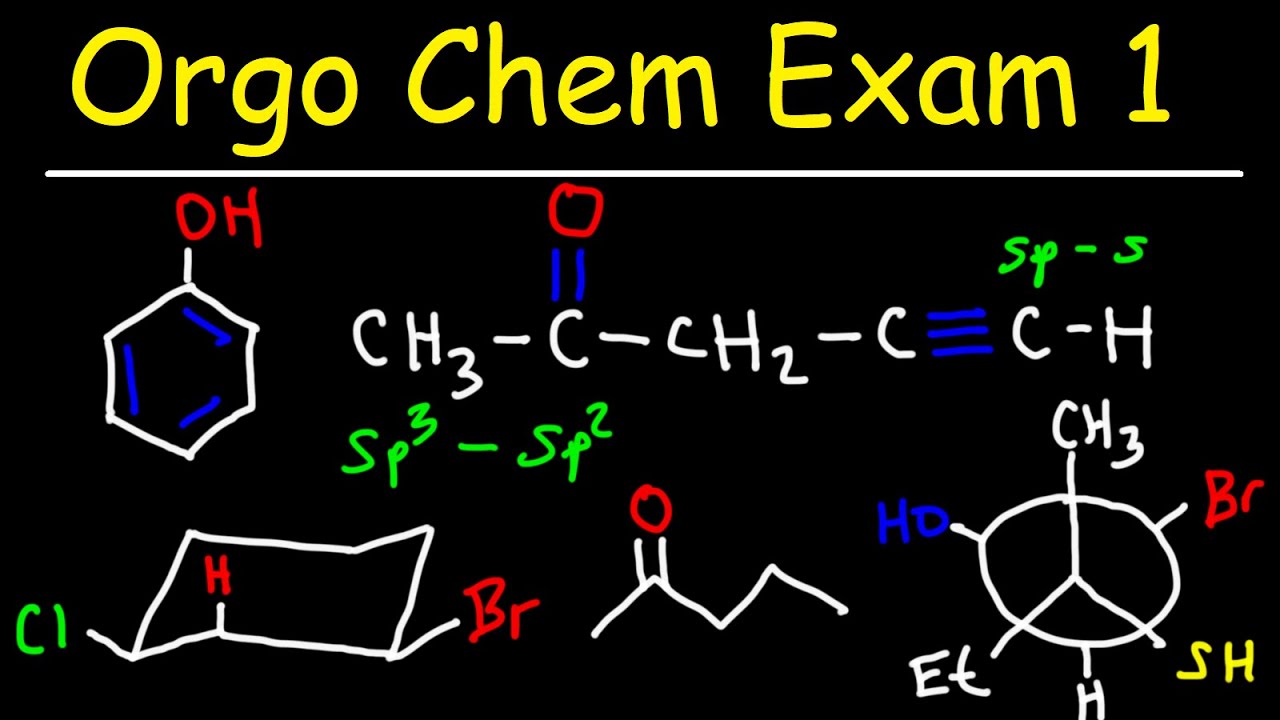
🧪 Functional Groups and Hybridization
This paragraph introduces the concept of functional groups in organic chemistry, explaining the difference between various types of alcohols, aldehydes, ketones, and carboxylic acids. It also covers the naming conventions for these groups and provides examples of how to draw and name Lewis structures. The paragraph further discusses hybridization, explaining how to determine the hybridization state of carbon atoms based on their steric number and the resulting bond angles and molecular geometry.
🔬 Understanding Bond Strength and Length
The paragraph explores the relationship between bond strength, bond length, and hybridization. It explains that triple bonds are stronger but shorter than double bonds, which in turn are stronger and shorter than single bonds. The paragraph uses examples to illustrate how the strength and length of bonds can be determined by the type of hybridization involved, such as SP, SP2, and SP3 hybridized carbons.
🔋 Formal Charge and Resonance Structures
This paragraph discusses the concept of formal charge, providing a formula for calculating it and applying it to various atoms in different molecules. It also introduces resonance structures, explaining how to draw them and identify the major resonance contributor. The paragraph emphasizes the importance of understanding electron flow and the stability of resonance structures in organic chemistry.
📉 Resonance Hybrids and Charge Distribution
The paragraph continues the discussion on resonance, focusing on the concept of resonance hybrids and how they represent the average structure of a molecule. It explains how to draw resonance hybrids by indicating the shared nature of pi bonds and partial charges among atoms. The paragraph uses examples to illustrate how resonance hybrids are derived from resonance structures and how they reflect the stability of a molecule.
🌐 Bond Angles, Geometry, and Electronegativity
This paragraph examines the relationship between hybridization, bond angles, and molecular geometry. It provides a detailed explanation of the typical bond angles associated with SP, SP2, and SP3 hybridized carbons, and the corresponding molecular geometries. The paragraph also touches on the concept of electronegativity, explaining how it affects the distribution of charge in molecules and the stability of resonance structures.
🚀 Advanced Topics in Organic Chemistry
The final paragraph wraps up the video script by summarizing advanced topics in organic chemistry, such as amines, amides, alkyl halides, alkenes, alkynes, and aromatic rings. It also mentions other functional groups like anhydrides and ethers, and encourages viewers to explore more examples of functional groups in a dedicated video on unusual functional groups. The paragraph concludes by highlighting the importance of understanding hybridization and bond angles in organic chemistry.
Mindmap
Keywords
💡Alkanes
💡IUPAC Nomenclature
💡Substituents
💡Functional Groups
💡Hybridization
💡Bond Angles
💡Formal Charge
💡Resonance Structures
💡Electronegativity
💡Sigma and Pi Bonds
Highlights
Introduction to alkanes and their naming conventions up to decane.
Explanation of IUPAC nomenclature for organic compounds with examples.
Demonstration of how to name alkanes with substituents like methyl, ethyl, propyl, isopropyl, sec-butyl, and ter-butyl groups.
Guidelines for numbering parent chains to give the lowest numbers for substituents.
Procedure for naming complex alkanes with multiple substituents, including the use of commas and hyphens.
Alphabetical order of substituents in alkane nomenclature and its significance.
Identification of the longest carbon chain for alkane nomenclature and handling of chains of equal length.
Introduction to functional groups in organic chemistry, including alcohols, aldehydes, ketones, and carboxylic acids.
Differentiation between primary, secondary, and tertiary alcohols and amines.
Explanation of the naming of esters, amides, and alkyl halides.
Overview of alkene and alkyne functional groups, including their structures and bonding.
Introduction to aromatic rings and their significance in organic chemistry.
Discussion on hybridization of carbon atoms and how to determine it using the steric number.
Correlation between hybridization and bond angles, including linear, trigonal planar, and tetrahedral geometries.
Explanation of bond strength and length, and how they relate to the type of bond (single, double, triple).
Calculation of formal charge on atoms and its importance in understanding molecular structures.
Introduction to resonance structures, how to draw them, and the concept of major and minor contributors.
Illustration of drawing resonance hybrids to represent the average structure of a molecule.
Transcripts
Browse More Related Video

Organic Chemistry 1 Final Exam Review
Organic Chemistry Exam 1 - IUPAC Nomenclature, Resonance, Acids & Bases, Newman Projections

Organic Chemistry - Basic Introduction

1.3 Valence Bond Theory and Hybridization | Organic Chemistry

IUPAC Nomenclature of Organic Chemistry

Organic Chemistry 1 Exam 2 Review Questions
5.0 / 5 (0 votes)
Thanks for rating: