Organic Chemistry 1 Exam 2 Review Questions
TLDRThis educational video script is tailored for students preparing for Organic Chemistry 1, specifically for Exam 2. It comprehensively covers key topics including stereochemistry, SN2, SN1, E1, and E2 reactions, as well as reactions involving alkenes and alkynes. The script provides detailed explanations on how to name compounds, determine configurations at chiral centers, and predict major substitution and elimination products. It also clarifies misconceptions about reaction rates and introduces various reagents used in converting alkanes to alkenones. The summary serves as a concise guide for students to navigate complex organic chemistry concepts.
Takeaways
- 🧪 Stereochemistry is a key topic for Organic Chemistry 1 students preparing for Exam 2, including SN2, SN1, E1, and E2 reactions, as well as alkene and alkyne reactions.
- 🔍 When naming compounds, focus on the configuration at each chiral center to determine if it's R or S, and consider the atomic number and carbon connectivity to assign group priorities.
- 📚 For stereochemistry problems, visualize the molecule's chiral centers and the direction of groups around them to determine the correct configuration (R or S).
- 🌟 Hydroboration-oxidation is a reaction that proceeds with Markovnikov's regiochemistry, placing the nucleophile on the less substituted carbon of the double bond.
- ⚠️ Be familiar with various reactions, as they may appear on exams, including oxymercuration-demercuration, which proceeds with Markovnikov regiochemistry without rearrangement.
- 🔑 Understanding reaction mechanisms is crucial, especially for SN1 and E1 reactions, which often occur together and are influenced by temperature.
- 🔄 Ring expansions are common in unstable four-carbon rings, which can expand to form more stable five- or six-carbon rings.
- 📉 The rate of SN1 and E1 reactions depends solely on the concentration of the substrate, not the nucleophile or base, as they are not involved in the slow, rate-determining step.
- 🛠️ For converting alkynes to ketones, reactions involving mercury sulfate with sulfuric acid and water can be used, proceeding with Markovnikov's regiochemistry and leading to tautomerization into the desired ketone.
- 🔬 In reactions with multiple functional groups, pay attention to intermolecular reactions and the specific behavior of each group in the presence of reagents.
- 📐 Chirality in molecules is determined by the absence of a plane of symmetry and the presence of chiral centers with four different groups attached.
Q & A
What topics are covered in the video for Organic Chemistry 1 students preparing for their second exam?
-The video covers stereochemistry, SN2, SN1, E1, E2 reactions, alkene reactions, and alkyne reactions.
How is the compound with a six-membered ring and a bromine on one carbon and a methyl on another named according to the video?
-The compound is named as 1-bromo-2-methyl cyclohexane.
What is the configuration (R or S) of a chiral center with a bromine atom and a methyl group attached to a carbon?
-The configuration is determined by the atomic numbers of the substituents and their positions. If the groups are arranged from highest to lowest priority (bromine, tertiary carbon, secondary carbon, hydrogen) in a clockwise direction, it results in the R configuration.
Why does the video emphasize the importance of determining the configuration at each chiral center in organic chemistry?
-The configuration at chiral centers is crucial because it affects the three-dimensional arrangement of atoms in a molecule, which in turn influences the molecule's properties and reactivity.
What is the role of the bromine atom in the compound discussed in the video?
-The bromine atom acts as a substituent in the compound, influencing its reactivity and the configuration at the chiral center it is attached to.
Which reagent will convert 3-methyl-1-butene to 3-methyl-2-butanol according to the video?
-Mercury acetate with water followed by sodium borohydride, through the oxymercuration-demercuration reaction, will achieve this conversion.
What is the significance of Markovnikov's rule in the context of the reactions discussed in the video?
-Markovnikov's rule predicts the regioselectivity of addition reactions to alkenes, stating that the hydrogen atom of the adding group will be attached to the carbon with the fewer hydrogen atoms (the less substituted carbon).
How does the video explain the difference between anti-addition and syn-addition in reactions involving alkenes?
-Anti-addition refers to the addition of two groups across a double bond such that they are opposite to each other, while syn-addition means they are on the same side.
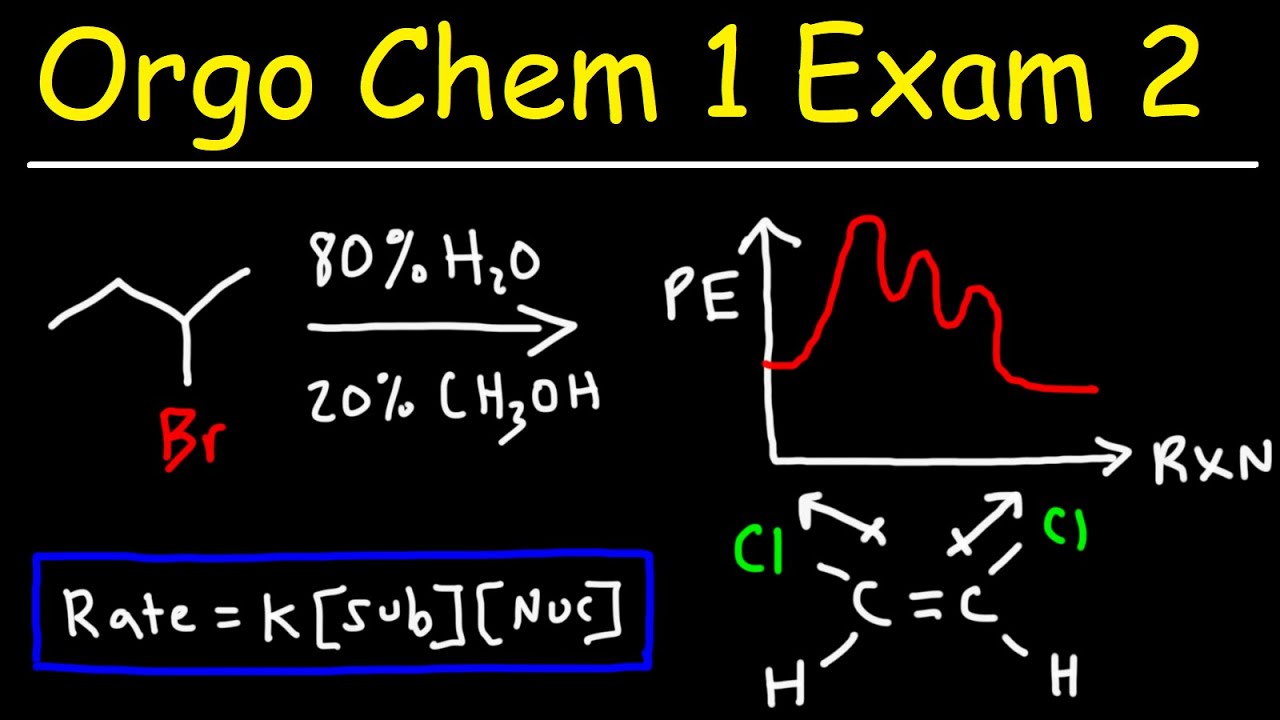
What is the major substitution product of the reaction between a secondary alkyl halide and methanol as described in the video?
-The major product is an ether, specifically 1-methoxy-1-methyl cyclopentane, formed through a solvolysis reaction involving an SN1 mechanism.
Why does the video mention that the rate of an SN1 reaction does not depend on the concentration of the nucleophile?
-The rate of an SN1 reaction is determined by the slowest step in the reaction mechanism, which involves the substrate and the leaving group, not the nucleophile. The nucleophile only reacts in the second, faster step.
How does the video explain the concept of chirality in molecules?
-Chirality is the property of a molecule that makes it non-superimposable on its mirror image. The video explains that a molecule is chiral if it lacks a plane of symmetry and has at least one chiral center, which is a carbon atom bonded to four different groups.
What is the major product of the reaction between 2-bromo-4-fluoropentane and potassium cyanide in hexamethyl phosphoramide (HMPA) as described in the video?
-The major product is the result of an SN2 reaction where the bromine is replaced by the cyanide group with inversion of configuration, leading to the formation of 2-cyano-4-fluoropentane.
How does the video describe the reaction of 1-methylcyclohexene with hydrogen using a palladium catalyst?
-The reaction converts the alkyne all the way to an alkane, not stopping at the alkene level, using hydrogen gas and a palladium catalyst.
What is the major product of the reaction involving 1-butyne and a reagent that leads to the formation of 2-butenone as explained in the video?
-The major product is 2-butenone, formed through a reaction involving hydroboration-oxidation with R2BH and subsequent tautomerization to convert the enol intermediate into a ketone.
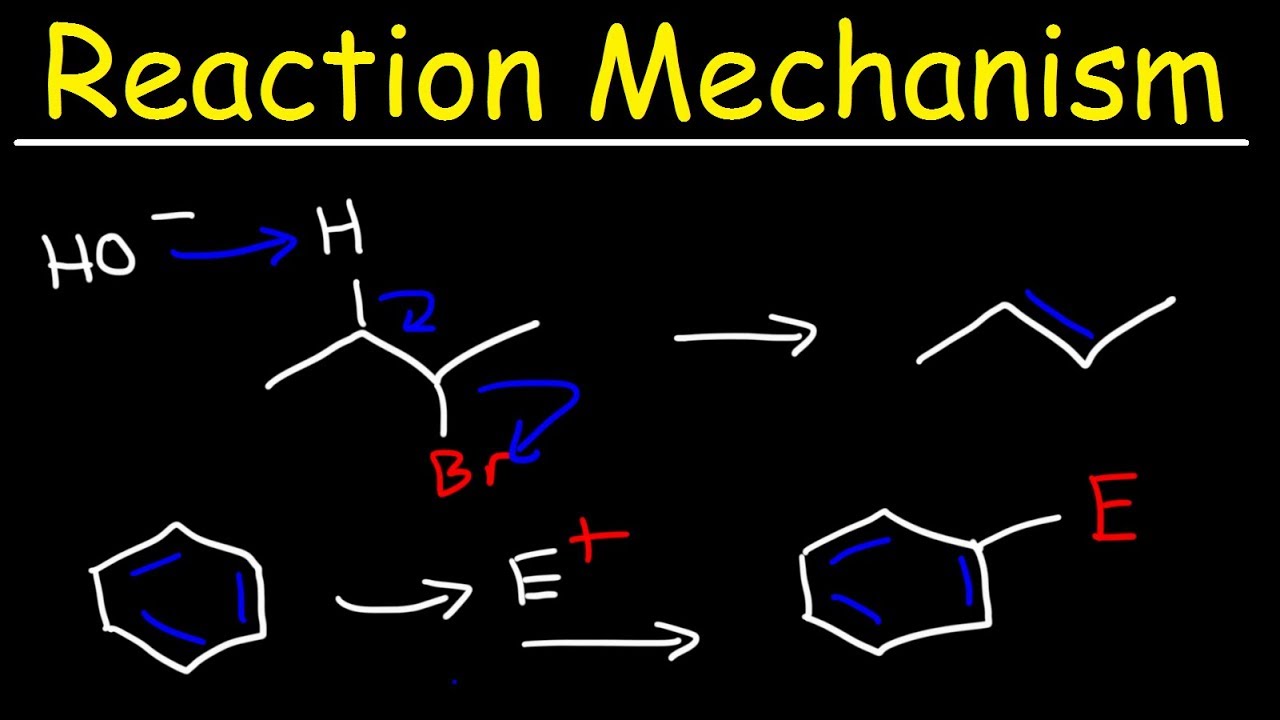
How does the video explain the concept of stereospecificity in the context of the E2 reaction shown?
-Stereospecificity means that the reaction occurs in a specific way with respect to the spatial arrangement of the groups involved. In the E2 reaction, hydroxide abstracts a proton from a carbon that is anti to the bromine, leading to the formation of a double bond with the methyl groups remaining on the same side.
Outlines
📚 Organic Chemistry Exam 2 Study Guide
This paragraph introduces a study aid for Organic Chemistry 1 students preparing for their second exam. It outlines the topics covered, including stereochemistry, SN2, SN1, E1, E2 reactions, alkene, and alkyne reactions. The speaker begins by addressing a question on naming a compound with a specific structure, focusing on the configuration at chiral centers to determine R or S configurations, using the Cahn-Ingold-Prelog priority rules and the clockwise/counterclockwise approach to assign the correct configuration.
🧪 Reagents for Converting 3-Methyl-1-Butene to 3-Methyl-2-Butanol
The speaker discusses the reagents that can convert 3-methyl-1-butene to 3-methyl-2-butanol. The explanation includes a step-by-step analysis of different reagents, such as BH3/THF with hydrogen peroxide, water, and sodium hydroxide (hydroboration oxidation), mercury acetate with water followed by sodium borohydride (oxymercuration-demercuration), and other options. The focus is on understanding the regiochemistry and mechanism behind each reaction to determine the correct reagent for the desired conversion.
🔍 Major Substitution Product of Alkyl Halide Reaction
This section delves into predicting the major substitution product when an alkyl halide reacts with methanol. The mechanism of the SN1 reaction is explored, including the formation of a secondary carbocation and the possibility of a carbocation rearrangement due to the presence of a tertiary carbon. The discussion also touches on ring expansions in carbocations and the concept of solvolysis, leading to the conclusion that the major product is 1-methoxy-1-methylcyclopentane.
⚗️ SN1 and E1 Reaction Rates and Conditions
The paragraph examines the relationship between reaction rates and the concentration of reactants in SN1 and E1 reactions. It clarifies that the rate of an SN1 reaction is independent of the nucleophile concentration, focusing instead on the substrate concentration. The explanation includes the rate law expressions for SN1 and E1 reactions and emphasizes the impact of temperature on the yield of substitution versus elimination products.
🔄 Conversion of Butane to Butenone Using Various Reagents
The speaker explores different reagents capable of converting butane to butenone, discussing the mechanisms and outcomes of each reaction. The reagents include hydrogen gas with a palladium catalyst, lithium metal in liquid methylamine, hydroboration oxidation with R2BH, and mercury sulfate with sulfuric acid and water. The correct reagent is identified as the one that leads to the formation of butenone through a series of steps involving enol and tautomerization.
🌀 Reaction of a Molecule with Multiple Functional Groups
This paragraph discusses the reaction involving a molecule with both alkene and alcohol functional groups. The focus is on the intermolecular reactions that can occur, particularly the reaction of the alkene with bromine to form a cyclic bromonium ion. The subsequent steps involve the attack of the oxygen atom on a tertiary carbon and the formation of a six-membered ring with an ether group, culminating in the final product after the removal of a hydrogen atom by a generic base.
💧 SN2 Reaction with Two Halogenated Pentane and Potassium Cyanide
The paragraph explains an SN2 reaction involving 2-bromo-4-fluoropentane, potassium cyanide, and hexamethylphosphoramide (HMPA) as the solvent. The conditions favor an SN2 reaction, and the discussion centers on the selection of the better leaving group between bromine and fluorine, leading to the displacement of bromine and the formation of a new compound with a cyanide group attached through inversion of stereochemistry.
🔍 Determining Chirality in Molecules
This section focuses on identifying chiral molecules among the given options. The explanation involves looking for a plane of symmetry and the presence of chiral centers. Molecules with a plane of symmetry or identical groups on one side are not chiral. The correct answer is identified as the molecule without a plane of symmetry and with distinct groups on each side, indicating chirality.
🌐 High Dilution Oxidation of Cyclohexene
The paragraph describes the high dilution oxidation of cyclohexene, which proceeds with anti-Markovnikov regiochemistry. The reaction involves the addition of BD3, followed by hydrogen peroxide and hydroxide, resulting in the formation of a hydroxyl group and deuterium on the same side of the molecule. The correct product is identified by ensuring that the hydroxyl group is on the less substituted carbon and is anti with respect to the methyl group but syn with respect to deuterium.
⏩ E2 Reaction with Stereospecificity
This section discusses a stereospecific E2 reaction where hydroxide abstracts a proton from a carbon adjacent to a bromine atom, leading to the formation of a double bond. The reaction results in the formation of a specific stereoisomer due to the presence of only one hydrogen atom that can be abstracted. The final product is identified as an E isomer, with the methyl and ethyl groups on the same side, and the hydroxyl group and remaining hydrogen on opposite sides.
Mindmap
Keywords
💡Organic Chemistry
💡Stereochemistry
💡SN2 Reaction
💡SN1 Reaction
💡E1 and E2 Reactions
💡Alkene Reactions
💡Alkyne Reactions
💡Chiral Center
💡Regiochemistry
💡Carbocation
💡Enol and Tautomerization
Highlights
The video is tailored for students preparing for Organic Chemistry 1 exam number two, covering key topics such as stereochemistry, SN2, SN1, E1, E2 reactions, alkene, and alkyne reactions.
Explanation of how to name a compound with a six-membered ring, cyclohexane, and determine the configuration at chiral centers using R/S notation.
Guide on identifying the priority of groups attached to a chiral carbon, including bromine, hydrogen, and carbons with varying levels of substitution.
Clarification on the difference between clockwise and counterclockwise directions when assigning R/S configurations to chiral centers.
Discussion on the conversion of 3-methyl-1-butene to 3-methyl-2-butanol using specific reagents and the importance of regiochemistry in the reactions.
Detailed analysis of hydroboration-oxidation, oxymercuration-demercuration, and other reactions for converting alkenes to alcohols with correct stereochemistry.
Mechanism explanation for the conversion of an alkene to an alcohol using water and sulfuric acid, highlighting potential rearrangements and carbocation stability.
Description of the epoxidation reaction using peroxy acid and the subsequent opening of the epoxide ring to form a diol with anti-addition.
Illustration of the solvolysis reaction in SN1 mechanism, including carbocation rearrangement and the role of the solvent as a nucleophile.
Analysis of the major substitution product for a secondary alkyl halide reacting with methanol, emphasizing the conditions that favor SN1 over E1 reactions.
Clarification on the rate laws for SN2, E1, and E2 reactions, and the impact of nucleophile and substrate concentrations on reaction rates.
Identification of false statements regarding reaction rates, particularly for SN1 and E1 reactions, and the role of the slow step in determining the rate.
Explanation of the conversion of butane to butenone using various reagents, focusing on the selectivity of the reactions for specific products.
Guide on predicting the major product of reactions involving molecules with multiple functional groups, such as alkenes and alcohols.
Discussion on the stereospecificity of reactions, particularly the formation of a specific E or Z isomer in E2 reactions.
Illustration of the major product formation in reactions with chiral molecules, including the importance of stereochemistry and the role of nucleophiles.
Identification of chiral molecules among given options by analyzing symmetry and the presence of chiral centers.
Prediction of the major product of reactions involving 1-methylcyclohexene and high-valent oxidation, emphasizing regiochemistry and syn addition.
Explanation of the reaction mechanism leading to the formation of a specific stereoisomer in an E2 reaction, considering the anti relationship between groups.
Transcripts
Browse More Related Video
Organic Chemistry 1 Exam 2 Review

Choosing Between SN2, SN1, E2 and E1 Reactions

Organic Chemistry Reactions Summary

E1 and E2 Reactions: Crash Course Organic Chemistry #22
Organic Chemistry - Reaction Mechanisms - Addition, Elimination, Substitution, & Rearrangement

Chem 51A 11/09/09 Ch. 6. Introduction to Understanding Organic Reactions
5.0 / 5 (0 votes)
Thanks for rating: