Organic Chemistry - Basic Introduction
TLDRThis video delves into the fundamentals of organic chemistry, focusing on carbon compounds and the importance of understanding bond formation with other elements. It explains the concept of Lewis structures, the differences between polar and non-polar bonds, and the significance of hydrogen bonding. The video also covers ionic bonds versus covalent bonds, the naming of alkanes, and the role of hybridization in determining bond types. Additionally, it explores the calculation of formal charge and the identification of functional groups in organic compounds, providing a solid foundation for students new to the subject.
Takeaways
- 📚 Organic chemistry focuses on carbon-containing compounds, important for college students in their first semester.
- 🔍 Carbon atoms prefer to form four bonds, while other elements like hydrogen, nitrogen, and halogens have different bonding preferences.
- 📈 Understanding bonding preferences is crucial for drawing Lewis structures, which represent the valence electrons and bonding in molecules.
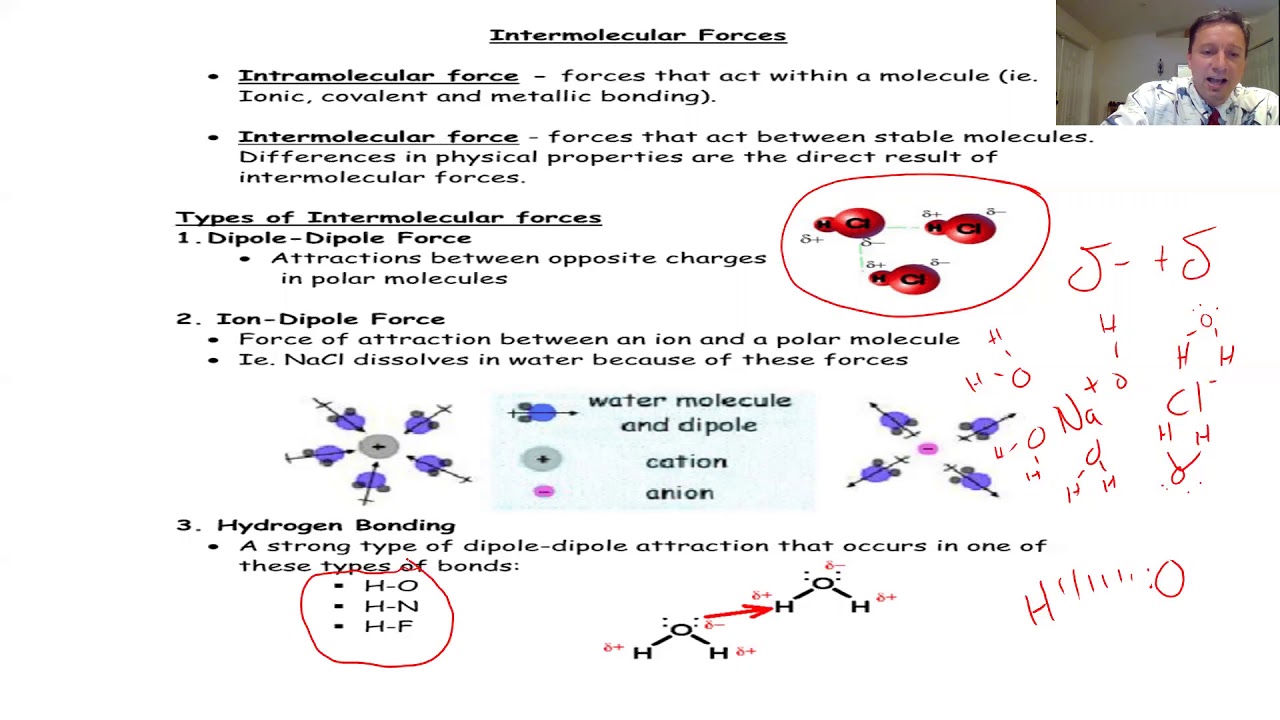
- 💧 The hydrogen bond in water (H2O) results from the bond between hydrogen and oxygen, and it explains water's high boiling point.
- 🔌 Polar covalent bonds occur when there's a significant electronegativity difference between bonded atoms, leading to charge separation.
- 🔄 Non-polar covalent bonds involve atoms with similar electronegativities, resulting in equal electron sharing, like in hydrocarbons.
- 🔗 Ionic bonds form through the transfer of electrons from metals to non-metals, creating oppositely charged ions that attract each other.
- 📊 The length and strength of bonds vary; generally, triple bonds are shortest and strongest, while single bonds are longest and weakest.
- 🌐 Hybridization of carbon atoms can be determined by the number of atoms and lone pairs surrounding it, affecting the shape of molecules.
- 🔢 Formal charge calculations help in understanding the distribution of electrons in a molecule and can be calculated using the formula: formal charge = valence electrons - (number of bonds + lone pairs).
- 🍷 Functional groups like alcohols, aldehydes, ketones, esters, and carboxylic acids define the properties and names of organic compounds.
Q & A
What is the focus of organic chemistry?
-Organic chemistry focuses on organic compounds, which are compounds that contain carbon atoms.
How many bonds does carbon typically form?
-Carbon typically forms four bonds due to having four valence electrons.
What is the role of hydrogen bonding in water's properties?
-Hydrogen bonding, which occurs between hydrogen and oxygen or nitrogen, explains why water has such a high boiling point.
What is the difference between polar and nonpolar covalent bonds?
-Polar covalent bonds occur when there is a significant electronegativity difference between the bonded atoms, leading to a charge separation. Nonpolar covalent bonds occur when the electronegativity difference is small or nonexistent, resulting in an even distribution of electron density.
How do you determine the hybridization of a carbon atom in a molecule?
-The hybridization of a carbon atom can be determined by counting the number of atoms and lone pairs attached to it. For example, if a carbon atom is bonded to four other atoms or has three groups around it, it is sp3 hybridized.
What is the general formula for naming alkanes?
-The general formula for naming alkanes is CnH2n+2, where 'n' represents the number of carbon atoms in the molecule.
What is the difference between a carbon-carbon single bond and a double bond in terms of bond length?
-A carbon-carbon single bond is longer than a double bond. The length of a carbon-carbon single bond is 154 picometers (1.54 angstroms), while a double bond is 133 picometers in ethene and 120 picometers in ethyne.
How do you calculate the formal charge of an atom?
-The formal charge of an atom is calculated as the number of valence electrons of the element minus the number of bonds and lone pairs (dots) around that atom.
What is the difference between an aldehyde and a ketone?
-An aldehyde contains a carbonyl group at the end of a chain, while a ketone has the carbonyl group in the middle of the chain.
How do you name a compound with a carboxylic acid functional group?
-A carboxylic acid is named by identifying the number of carbon atoms in the chain and using the suffix '-oic acid'. For example, a five-carbon carboxylic acid is called pentanoic acid.
What is the role of functional groups in determining the properties and reactivity of organic compounds?
-Functional groups are specific groups of atoms within a molecule that determine the molecule's chemical properties and reactivity. They are responsible for the characteristic behaviors and reactions of different types of organic compounds.
Outlines
📚 Introduction to Organic Chemistry
This paragraph introduces the basics of organic chemistry, focusing on the study of organic compounds that contain carbon atoms. It explains the bonding preferences of carbon and other elements, such as hydrogen, beryllium, boron, nitrogen, oxygen, and halogens. The importance of understanding these preferences is emphasized for drawing Lewis structures, with the example of water's Lewis structure provided. The concept of hydrogen bonding is introduced, explaining its impact on water's high boiling point. The paragraph also touches on polar and non-polar bonds, using the carbon-fluorine bond as an example of a polar covalent bond and contrasting it with the non-polar carbon-hydrogen bond.
🔍 Understanding Covalent and Ionic Bonds
This section delves deeper into the types of chemical bonds, differentiating between covalent and ionic bonds. Covalent bonds are further divided into nonpolar, polar, and hydrogen bonds, with examples provided for each. The paragraph also explains the concept of electronegativity and how it affects bond polarity. The formation of ionic bonds is described using the reaction between sodium and chlorine as an example, highlighting the transfer of electrons and the resulting electrostatic force that holds the ions together in an ionic crystal.
📈 Naming and Bond Characteristics in Alkanes
This paragraph discusses the nomenclature of alkanes, which are saturated organic compounds with carbon atoms fully bonded with hydrogen atoms. The general formula for alkanes is explained, and examples of alkanes from one to ten carbon atoms are provided. The paragraph then moves on to discuss the Lewis structures of ethane, ethene, and ethyne, explaining how to represent the different types of carbon-carbon bonds (single, double, and triple) and their common names. The characteristics of these bonds, including length and strength, are compared, with a focus on the significance of sigma and pi bonds.
🔬 Hybridization and Bond Order in Organic Compounds
This section introduces the concept of hybridization in carbon atoms within organic compounds, explaining how to determine the hybridization based on the number of atoms and lone pairs around a carbon atom. The strength of sigma and pi bonds is compared, and the bond order for single, double, and triple bonds is defined. The paragraph also discusses how to calculate the formal charge of an element using a specific formula, with examples for carbon atoms in different situations. The concept of radicals as neutral species with odd numbers of electrons is briefly mentioned.
🧪 Functional Groups and Nomenclature in Organic Compounds
This paragraph focuses on identifying and naming functional groups in organic compounds, starting with alcohols, aldehydes, ethers, ketones, esters, and carboxylic acids. The structural differences between aldehydes and ketones are highlighted, as well as the naming conventions for esters and carboxylic acids. The paragraph also provides guidance on how to expand condensed structures into full Lewis structures, emphasizing the placement of methyl and methylene groups in the molecule. The importance of understanding the bonding preferences of elements is reiterated to facilitate the drawing of Lewis structures and the identification of functional groups.
Mindmap
Keywords
💡Organic Chemistry
💡Carbon Bonds
💡Lewis Structures
💡Hydrogen Bond
💡Polar and Nonpolar Bonds
💡Ionic and Covalent Bonds
💡Alkanes
💡Alkenes and Alkynes
💡Bond Length and Bond Strength
💡Hybridization
💡Formal Charge
Highlights
Organic chemistry focuses on compounds containing carbon atoms.
Carbon likes to form four bonds, but the number of bonds other elements form is also important to know.
Understanding the bonding preferences of elements is crucial for drawing Lewis structures.
The Lewis structure of water (H2O) involves two lone pairs on oxygen to achieve an octet.
Hydrogen bonding, such as in H2O, explains the high boiling point of water.
Methyl fluoride's Lewis structure reflects the bonding preferences of carbon, hydrogen, and fluorine.
Carbon-fluorine bonds are polar due to the electronegativity difference between carbon and fluorine.
Alkanes are saturated organic compounds with a general formula of CnH2n+2.
Ethene (C2H4) and ethyne (C2H2) are examples of unsaturated compounds with double and triple bonds, respectively.
The length of carbon-carbon bonds varies with single bonds being the longest and triple bonds being the shortest.
The strength of a bond is determined by its type, with triple bonds being the strongest and single bonds the weakest.
Sigma bonds are stronger than pi bonds, which affects the overall strength of a molecule's bonding.
Bond order is a simple numerical representation of the number of bonds between two atoms.
Hybridization of carbon atoms can be determined by counting the number of atoms and lone pairs attached to it.
The hybridization of a bond can be deduced by examining the hybridization of the atoms it connects.
Calculating formal charge involves subtracting the number of bonds and lone pairs from the valence electrons of an element.
The presence of a functional group, such as OH for alcohols or CHO for aldehydes, defines the type of organic compound.
Naming organic compounds involves understanding their functional groups and following specific nomenclature rules.
Esters and ketones differ in the placement of their carbonyl group within the molecular structure.
Carboxylic acids are characterized by a carbonyl group combined with a hydroxyl group and are named based on the number of carbon atoms.
Expanding complex organic structures requires identifying the correct placement of functional groups and carbon chains.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: