The Discovery of Atomic Structure (Chemistry & Physics) - [1-2-5]
TLDRThis transcript details the historical journey and scientific advancements in understanding atomic structure. Starting from the ancient Greeks' thought experiments on the infinite divisibility of matter, it progresses through the development of atomic theory in the 1800s, led by figures like Dalton. The lecture highlights the discovery of radioactivity in uranium and other elements by scientists like Marie Curie, and the subsequent identification of three types of radiation: alpha, beta, and gamma. It then delves into the experimental work of Rutherford, which led to the realization that atoms have a concentrated nucleus with protons and neutrons, surrounded by a cloud of electrons. The script also touches on the quantum mechanical model of the atom, contrasting it with the earlier Bohr model, and explains the wave-particle duality of electrons as described by quantum mechanics. The summary underscores the complexity and counter-intuitive nature of quantum mechanics, which is fundamental to modern technology, and invites further exploration into the realm of chemistry and atomic interactions.
Takeaways
- 🧬 The journey of discovering atomic structure is a culmination of centuries of scientific inquiry, starting with ancient Greek thought experiments about the fundamental building blocks of matter.
- 📚 Dalton's atomic theory in the 1800s proposed that all substances are made of atoms, which combine in different ways to form different materials, much like Lego bricks.
- 🔬 The discovery of the electron through cathode rays and Millikan's oil drop experiments was a significant step in understanding the subatomic realm.
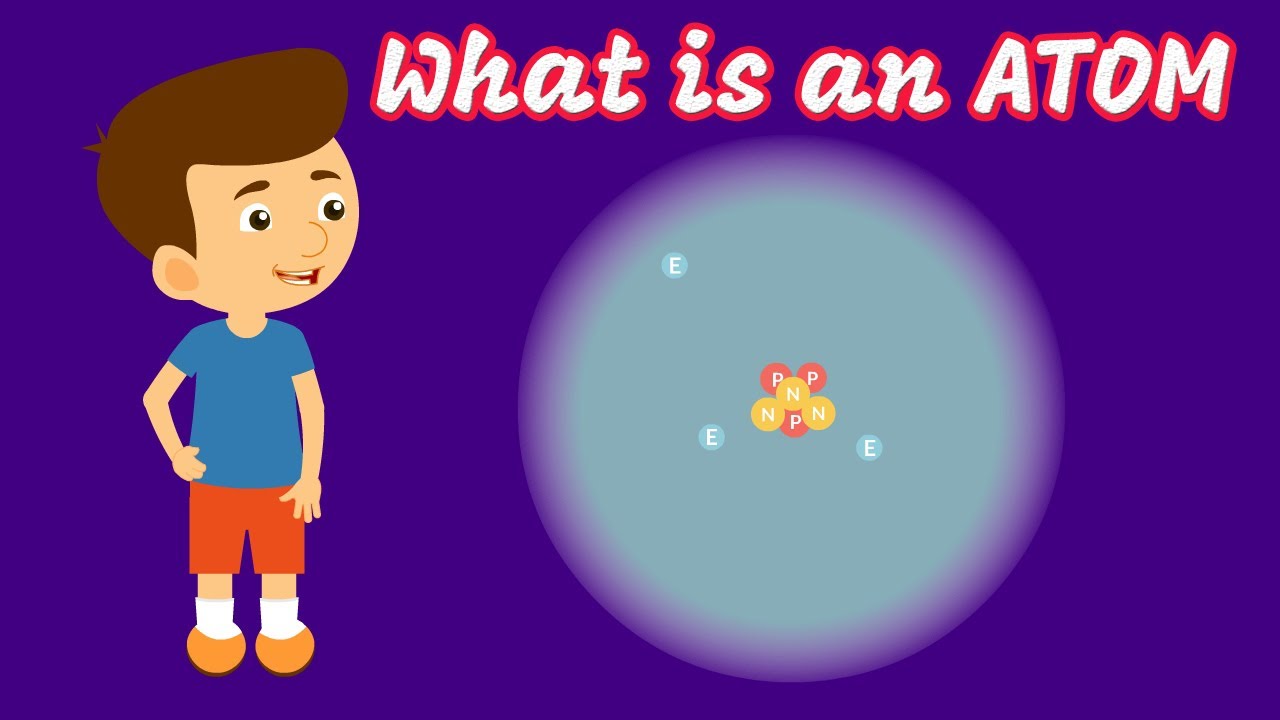
- ⚛️ Atoms are made of a nucleus, containing protons and neutrons, and electrons that orbit around it. The nucleus is very dense and contains most of the atom's mass.
- 🤔 The plum pudding model, an early and incorrect model of the atom, suggested that positive charge was spread evenly throughout the atom with electrons scattered within.
- 🎯 Rutherford's gold foil experiment with alpha particles led to the realization that atoms have a concentrated nucleus, which caused some alpha particles to bounce back.
- 🧲 The strong nuclear force, which is stronger than electromagnetic force, holds the nucleus together despite the repulsion between protons.
- ⚡ Gamma rays, beta particles, and alpha particles are three types of radiation discovered from radioactive materials, each with different properties and interactions with matter.
- 🌐 Quantum mechanics introduced the concept that electrons behave as both particles and waves, leading to the understanding of electron clouds or orbitals rather than fixed orbits.
- ⚛️ The modern atomic model includes the nucleus with protons and neutrons, and a cloud of probability where electrons are likely to be found, based on their wave functions.
- 🚀 Quantum mechanics is crucial for the development of modern technology, including computers, phones, and other electronics, and is our most accurate theory of the atomic and subatomic world.
Q & A
What was the prevailing idea among ancient Greeks regarding the fundamental building block of matter?
-The prevailing idea among ancient Greeks was that there would eventually be a fundamental building block of matter, which they called atoms, although they did not know what it looked like.
What is the basic principle behind chemical reactions?
-The basic principle behind chemical reactions is that different substances, which are different combinations of atoms, can be transformed into new substances by rearranging the atoms, similar to how a Lego set can be assembled in different ways.
How did the discovery of the electron contribute to the understanding of atomic structure?
-The discovery of the electron showed that atoms have smaller components, leading to the understanding that atoms are not indivisible. This discovery was a stepping stone to realizing that atoms have a complex internal structure, including a nucleus and orbiting electrons.
What are the three types of radiation discovered from radioactive materials, and how do they interact with charged plates?
-The three types of radiation are alpha particles, beta particles, and gamma particles. Alpha particles are positively charged and are deflected towards the negative plate. Beta particles are negatively charged and are attracted to the positive plate. Gamma particles have no charge and continue in a straight line without deflection.
What is the significance of the strong nuclear force in holding the nucleus together?
-The strong nuclear force is a powerful force that acts within the nucleus, holding protons and neutrons together. It is stronger than the electromagnetic force that would otherwise cause protons to repel each other due to their positive charges. This force is crucial for the stability of atoms, especially heavier ones.
How did the plum pudding model of the atom influence early atomic theory?
-The plum pudding model proposed that an atom was a blob of positive charge with negatively charged electrons scattered within it, similar to plums in a pudding. This model was influential as it was the first to localize the positive charge to the atom's center but was later disproved by Rutherford's gold foil experiment.
What was the conclusion of Rutherford's gold foil experiment regarding the atom's structure?
-Rutherford's experiment led to the conclusion that atoms have a concentrated center, or nucleus, where most of the atom's positive charge and almost all of its mass is located. This was a significant departure from the plum pudding model and led to the modern understanding of the atomic nucleus.
What is the role of quantum mechanics in understanding the behavior of electrons?
-Quantum mechanics describes the wave-like behavior of electrons, which cannot be accurately described by classical physics. It introduces concepts such as wave-particle duality, probability distributions, and the Heisenberg uncertainty principle, which together help explain why electrons do not simply fall into the nucleus.
Why don't electrons with negative charge crash into the positively charged nucleus?
-Electrons do not crash into the nucleus because of the probabilistic nature of their existence as described by quantum mechanics. Electrons are not in a fixed orbit but exist in a cloud of probability around the nucleus, with a highest likelihood of being found at certain distances, known as electron shells.
How does the Heisenberg uncertainty principle relate to the behavior of electrons in an atom?
-The Heisenberg uncertainty principle states that it is impossible to simultaneously know the exact position and momentum of a particle. Applied to electrons, this principle implies that if an electron were to be found at the nucleus (a precise position), it would have to have a very uncertain momentum, which would prevent it from staying there due to the high kinetic energy.
What is the significance of the discovery of the neutron in 1932?
-The discovery of the neutron completed the basic picture of the atomic nucleus, showing that atoms consist of protons, which have a positive charge, and neutrons, which have no charge. This discovery was crucial for understanding nuclear reactions, isotopes, and the stability of atomic nuclei.
Outlines
🌌 Introduction to Atomic Structure Discovery
The video introduces the topic of atomic structure, highlighting the historical quest to understand the composition of matter. It discusses ancient Greek thought experiments on the divisibility of matter and the development of atomic theory by scientists like Dalton in the 1800s. The importance of atoms in forming the substances of the universe, including living organisms, is emphasized. The video sets the stage for a deeper exploration into the subatomic realm and the structure of the atom's nucleus.
🔬 Early Experiments with Radioactivity
This paragraph delves into the discovery of radioactivity and the experimental methods used to study it. It describes how scientists observed that radioactive materials could expose photographic film, leading to the understanding that radiation could pass through solid objects. The narrative focuses on experiments conducted with uranium and other elements, using lead blocks and charged plates to detect and analyze the behavior of different types of radiation, such as gamma, beta, and alpha particles.
📡 Understanding the Different Types of Radiation
The video explains the three types of radiation discovered through experiments: gamma, beta, and alpha particles. It details how gamma particles, being uncharged, travel in a straight line, while beta particles, with a negative charge, and alpha particles, with a positive charge, are deflected by charged plates. The properties of these particles, including their charge and mass, are discussed, leading to the current understanding of gamma rays as high-energy electromagnetic waves, beta particles as electrons, and alpha particles as clusters of two protons and two neutrons.
⚛️ The Composition and Forces within the Atom
This section explores the composition of the atom, focusing on the nucleus and the strong nuclear force that holds protons and neutrons together. It challenges the notion that like charges repel by explaining the strong nuclear force, which is stronger than the electromagnetic force but acts over a very short distance. The video also touches on the concept of radioactive decay in heavy elements, where the nucleus becomes unstable and undergoes decay due to the strong nuclear force being insufficient to hold the large nucleus together.
🎼 The Plum Pudding Model and its Disproof
The video discusses the historical plum pudding model of the atom, which proposed that positive charges were evenly distributed throughout the atom with electrons scattered within. It then describes how experiments, particularly those conducted by Rutherford using gold foil and alpha particles, disproved this model. The unexpected results, such as alpha particles bouncing back, led to the proposal of a concentrated atomic nucleus containing most of the atom's mass, with electrons surrounding it.
🌟 The Wave-Particle Duality and Quantum Mechanics
The video concludes with a discussion on the wave-particle duality of electrons and the principles of quantum mechanics. It explains that electrons are not small balls orbiting the nucleus but rather wave-like entities existing in a probability cloud around the atom. The concept of electron probability distribution is introduced, with the electron most likely to be found at a certain distance from the nucleus, as described by the wave function. The video emphasizes the complexity and counter-intuitive nature of quantum mechanics, which is essential for understanding the behavior of atoms at the most fundamental level.
Mindmap
Keywords
💡Atomic Structure
💡Chemical Reactions
💡Subatomic Particles
💡Radioactivity
💡Plum Pudding Model
💡Rutherford's Gold Foil Experiment
💡Quantum Mechanics
💡Electron
💡Proton
💡Neutron
💡Wave-Particle Duality
Highlights
The lesson summarizes the culmination of thousands of years of scientific inquiry into the structure of the universe and the nature of matter.
Ancient Greeks performed thought experiments to deduce the existence of a fundamental building block of matter, which we now call atoms.
John Dalton and others proposed the atomic theory of matter in the 1800s, suggesting everything is made of different combinations of atoms.
Chemical reactions involve the rearrangement of atoms to form new substances with different properties.
The discovery of the electron through cathode rays and Millikan's oil drop experiments was a significant step in understanding the atomic structure.
The realization that atoms have subatomic particles inside them, such as electrons, led to a deeper understanding of the atom's core, known as the nucleus.
Radioactivity was discovered with uranium, which emits high-energy radiation, sparking further investigation into the nature of radiation and atomic structure.
Experiments with charged plates and radiation led to the identification of three types of radiation: alpha, beta, and gamma particles.
Gamma particles are high-energy electromagnetic waves, beta particles are negatively charged electrons, and alpha particles are positively charged with double the mass of a proton.
The strong nuclear force, stronger than electromagnetic forces, holds the nucleus together but only acts over very short distances.
Rutherford's gold foil experiment with alpha particles led to the discovery that atoms have a concentrated nucleus, contradicting the plum pudding model.
The atom is mostly empty space with the mass concentrated in the nucleus, and electrons existing in a cloud-like distribution around it.
The Bohr model of the atom was developed, suggesting electrons orbit the nucleus at certain distances or shells, which could explain atomic spectra.
Quantum mechanics introduced the concept that electrons behave as both particles and waves, leading to the wave-particle duality.
Electrons are described by wave functions, which represent the probability of finding an electron in a particular location around the nucleus.
The Heisenberg uncertainty principle states that the position and momentum of an electron cannot both be precisely known at the same time.
Electrons have a small but non-zero probability of being found at any location in the universe, including inside the nucleus.
The journey from early atomic models to quantum mechanics illustrates the iterative process of scientific discovery and the evolution of our understanding of the atom.
Quantum mechanics is crucial for modern technology, including the development of computers, phones, and other electronic devices.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: