Lesson 14 - Ionic Compounds in Chemistry
TLDRThe chemistry tutor video script delves into the topic of ionic compounds, which are formed by the union of metals from the left side of the periodic table and nonmetals from the right side. Metals tend to lose electrons to resemble noble gases, becoming positively charged ions, while nonmetals gain electrons to achieve a noble gas configuration, becoming negatively charged. This fundamental difference in electron affinity results in an electrostatic attraction between the oppositely charged ions, leading to the formation of stable ionic compounds. A common example of such a compound is an oxide, like rust (iron oxide), which occurs when metals react with oxygen in the atmosphere. These compounds do not exist as discrete molecules but are arranged in a regular, crystalline lattice structure, as seen in table salt (sodium chloride) under a microscope, where alternating sodium and chlorine ions form a repeating pattern.
Takeaways
- 🌟 Ionic compounds are formed from the combination of a metal (from the left side of the periodic table) and a nonmetal (from the right side).
- 🔋 Metals tend to lose electrons to resemble noble gases, becoming positively charged ions.
- ⚡ Nonmetals prefer to gain electrons to achieve a noble gas electron configuration, resulting in negatively charged ions.
- 🧲 Oppositely charged ions attract each other, leading to the formation of ionic bonds.
- 🔑 The periodic table's layout helps predict the charge of metals on the left and nonmetals on the right, although it's more predictable for metals on the far left.
- 🌌 Transition metals in the center of the periodic table also tend to lose electrons but their charges are less predictable.
- 🍬 Ionic compounds often form a crystalline or lattice structure, as opposed to existing as individual molecules.
- 🧊 Oxides are a common type of ionic compound, formed when metals react with oxygen in the atmosphere.
- 🍽 An everyday example of an ionic compound is table salt (sodium chloride), which has a regular, repeating crystal structure.
- 🔬 Under a microscope, the arrangement of ions in ionic compounds like sodium chloride would appear in a pattern, with alternating positive and negative ions.
- 📐 The term 'lattice structure' describes the regular, three-dimensional arrangement of atoms in ionic compounds.
Q & A
What are ionic compounds?
-Ionic compounds are formed when a metal from the left-hand side of the periodic table combines with a nonmetal from the right-hand side. They are made up of positive and negative ions that are attracted to each other due to their opposite charges.
Why do metals tend to lose electrons?
-Metals tend to lose electrons because they want to achieve a stable electron configuration similar to that of a noble gas. This is often by losing electrons to become positively charged ions.
How do nonmetals typically behave in terms of electron behavior?
-Nonmetals typically gain electrons to complete their outer electron shell and achieve a stable noble gas configuration, resulting in a negative charge.
What is the role of the zigzag line on the periodic table?
-The zigzag line on the periodic table separates metals, which are generally found on the left, from nonmetals, which are on the right. This line helps to predict the tendency of elements to lose or gain electrons.
Why are transition metals harder to predict in terms of their ionic charge?
-Transition metals, located in the central block of the periodic table, can have multiple oxidation states, making it more challenging to predict their ionic charge without referring to a resource or learning them over time.
What is an example of an ionic compound?
-An example of an ionic compound is iron oxide, commonly known as rust. It forms when iron reacts with oxygen in the atmosphere.
How do ionic compounds typically exist?
-Ionic compounds typically exist in a lattice structure, where the positively charged metal ions and negatively charged nonmetal ions are arranged in a regular, three-dimensional pattern.
What is the binding force in ionic compounds?
-The binding force in ionic compounds is electrostatic attraction, which is the force between the oppositely charged ions.
Why are ionic compounds often crystalline in structure?
-Ionic compounds are often crystalline because the regular arrangement of ions in a lattice structure leads to a crystalline appearance and properties such as shininess and a fixed melting point.
What is the chemical formula for table salt?
-The chemical formula for table salt is NaCl, indicating a compound formed from sodium (Na) and chlorine (Cl) ions.
How does the formation of an oxide layer on metal surfaces occur?
-The formation of an oxide layer on metal surfaces occurs when metals are exposed to oxygen in the atmosphere, leading to a chemical reaction that forms a metal oxide layer on the surface.
Why do some metals tarnish or change color when exposed to air?
-Some metals tarnish or change color when exposed to air due to the formation of an oxide layer. This is a result of the metal reacting with oxygen to form a metal oxide, which can have different visual properties than the original metal.
Outlines
🔬 Introduction to Ionic Compounds
This paragraph introduces the concept of ionic compounds, which are formed by the combination of a metal (typically found on the left side of the periodic table) and a nonmetal (typically found on the right side). The paragraph explains that metals tend to lose electrons to achieve a noble gas configuration, thus becoming positively charged ions, while nonmetals gain electrons to achieve the same, becoming negatively charged ions. The electrostatic attraction between these oppositely charged ions results in the formation of ionic compounds. The paragraph also mentions that these compounds do not exist as discrete molecules but rather in a lattice structure, like a crystal, with a regular arrangement of atoms. An example given is sodium chloride (table salt), which has a crystalline structure.
Mindmap
Keywords
💡Ionic Compounds
💡Periodic Table
💡Ions
💡Noble Gas Configuration
💡Electrostatic Attraction
💡Lattice Structure
💡Transition Metals
💡Metals and Nonmetals
💡Oxide
💡Crystal
💡Sodium Chloride
Highlights
Introduction to ionic compounds, which are formed by the combination of a metal and a nonmetal from opposite sides of the periodic table.
Explanation of ions as charged atoms resulting from the loss or gain of electrons.
Metals on the left-hand side of the periodic table tend to lose electrons to resemble noble gases.
Nonmetals on the right-hand side of the periodic table tend to gain electrons to achieve a noble gas electron configuration.
Ionic compounds are formed through electrostatic attraction between positive and negative ions.
Common example of ionic compounds is oxides, such as rust (iron oxide), which form when metals react with oxygen in the atmosphere.
Ionic compounds do not exist as individual molecules but are arranged in a lattice or crystal structure.
Sodium chloride (table salt) is a typical example of an ionic compound with a crystalline structure.
Transition metals in the center of the periodic table also tend to lose electrons but their charge is less predictable.
The formation of ionic compounds involves metals becoming positive ions and nonmetals becoming negative ions.
Ionic compounds are stable due to the opposite charges of the ions attracting each other.
The periodic table's left-hand side metals have a predictable charge based on their column.
Nonmetals always want to gain electrons to become negatively charged ions.
When metals and nonmetals come together, they form ionic compounds through the transfer of electrons.
Ionic compounds are characterized by their crystalline, lattice-like structure where atoms are arranged in a regular pattern.
The shiny and crystalline appearance of sodium chloride is indicative of its ionic nature.
Ionic compounds are held together by the electrostatic forces between the ions.
The periodic table's structure helps predict the formation and charges of ionic compounds.
Transcripts
Browse More Related Video

What Are Ions | Properties of Matter | Chemistry | FuseSchool

Introduction to Ionic Bonding and Covalent Bonding

Lesson 15 - Ionic Compounds With Polyatomic Ions (Chemistry Tutor)

What is an Ion? Why Atoms Lose Their Electrons?

Ionic vs. Molecular
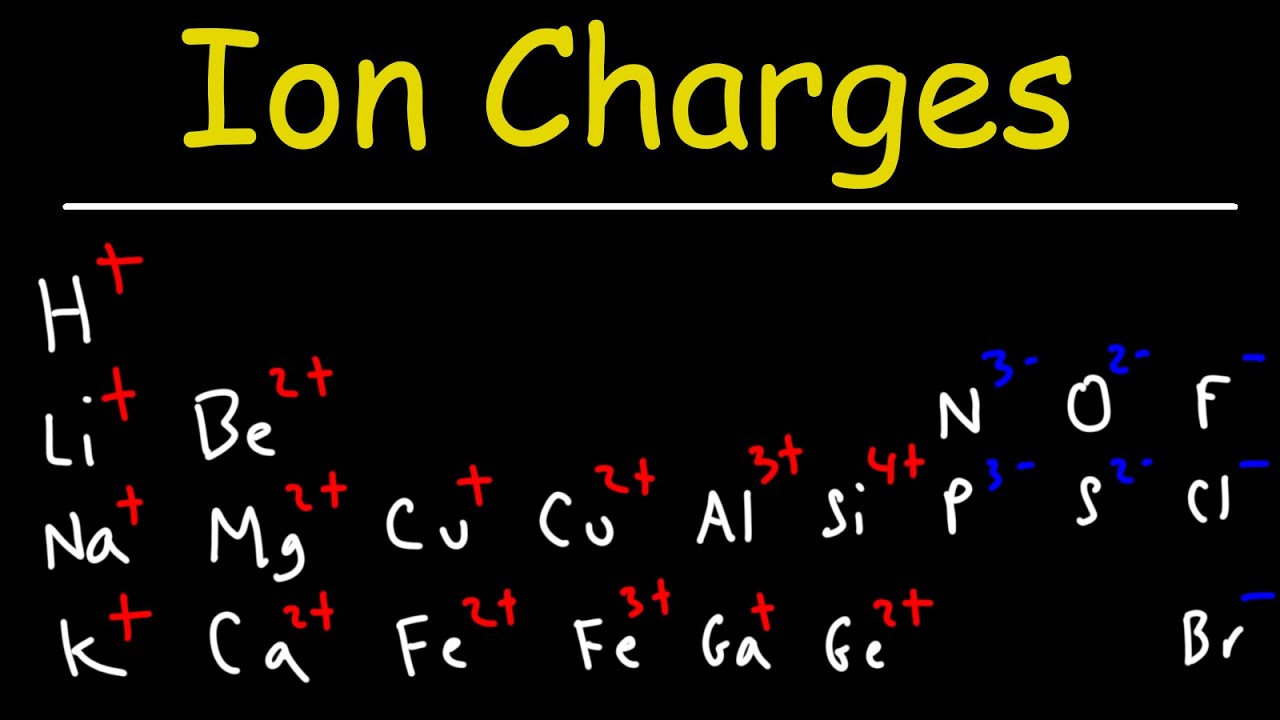
How To Determine The Charge of Elements and Ions - Chemistry
5.0 / 5 (0 votes)
Thanks for rating: