How to prepare a Serial Dilution
TLDRThe video script outlines the process of preparing a serial dilution, a common laboratory technique used for various purposes such as determining protein concentration or adjusting cell counts in a solution. The script explains that serial dilutions are typically either 10-fold or 2-fold, with a dilution factor representing the amount of content reduction at each step. The video demonstrates how to prepare a 10-fold serial dilution by adding stock solution to water in a stepwise manner, resulting in a decreasing concentration of the original substance. It also touches on the process for a 2-fold dilution, which involves equal parts of stock solution and water. The script emphasizes the importance of proper mixing and careful transfer between steps to achieve accurate dilutions. The video concludes by encouraging viewers to subscribe, engage with the content, and follow the channel on social media.
Takeaways
- 🧪 Serial dilution is a stepwise dilution process using a constant dilution factor, typically 10-fold or 2-fold.
- 📝 The dilution factor is crucial; for a 10-fold dilution, each step has 10 times less of the content than the previous one.
- 💧 The choice of diluent is important; it can be water or a specific medium depending on the application.
- 📏 To prepare a 10-fold dilution, fill the tubes to 90% of their capacity and add the stock solution to reach the desired dilution.
- 🔄 Proper mixing is essential after each addition of stock solution to ensure homogeneity.
- 📈 The process can be visualized as starting with a 1:10 dilution, then transferring 1ml to the next tube with 9ml of water for a 1:100 dilution, and so on.
- 🔬 Some assays require multiple steps, up to seven for determining bacterial concentrations, each with a 10-fold decrease.
- 📉 For a twofold dilution, equal volumes of the stock solution and diluent are mixed, and 1ml is transferred to the next tube.
- 🔁 The serial dilution process is repeated, maintaining the dilution factor with each step.
- ⚖️ Accurate measurement and careful resuspension are necessary to ensure the integrity of the dilution series.
- 📚 The script provides a clear, step-by-step guide on how to perform serial dilutions in a laboratory setting.
Q & A
What is a serial dilution?
-A serial dilution is a stepwise dilution by a constant dilution factor, commonly used in labs for various techniques and essays such as determining protein concentration or cell count.
What are the common dilution factors used in serial dilutions?
-The most common dilution factors in serial dilutions are 10-fold and 2-fold.
In the provided example, what is the dilution factor?
-The dilution factor in the provided example is 10-fold.
How is the first step of a 10-fold serial dilution prepared?
-The first step of a 10-fold serial dilution is to fill up the tubes to 9 out of 10 parts with water or a specific medium, and then add 1 part of the stock solution to reach a dilution of 1 to 10.
What is the total volume after adding the stock solution to the water in the first step?
-The total volume after adding the stock solution to the water in the first step is 10 mL.
How is the concentration of the dilution changed in the subsequent steps?
-In the subsequent steps, 1 mL of the previous dilution is mixed with 9 mL of water or medium, and this process is repeated to achieve the desired dilution factor.
What is the final concentration after the second step in the example?
-After the second step, the final concentration is 1 to 100.
How many steps are included in some essays for determining bacterial concentrations?
-Some essays for determining bacterial concentrations include up to seven steps, ranging from 10 to the power of -1 to 10 to the power of -7.
What changes when a twofold serial dilution is required?
-In a twofold serial dilution, the volume of the medium or water added is equal to the volume of the previous solution, meaning 1 mL of water is added to 1 mL of the stock solution, and 1 mL of this mixture is transferred to the next tube.
What is the purpose of mixing the dilution properly?
-Proper mixing ensures that the dilution is uniform and accurate, which is crucial for the reliability of the results in lab experiments.
Why is it important to subscribe to the channel and turn on notifications?
-Subscribing and turning on notifications helps to stay updated with the latest content, ensuring that viewers do not miss any new videos or important information shared by the channel.
How can viewers follow the lab's updates on social media?
-Viewers can follow the lab's updates on social media by following the Twitter account @henrics_lab.
Outlines
🔬 Understanding Serial Dilution Technique
The video script explains the concept and process of preparing a serial dilution, which is a stepwise dilution by a constant factor, commonly used in labs for various purposes like determining protein concentration or adjusting cell counts. It emphasizes the importance of deciding whether to dilute the content in water or a specific medium. The example given is a 10-fold serial dilution, where each step contains 10 times less of the content than the previous one. The process is demonstrated by adding 1 ml of stock solution to 9 ml of water to achieve a 1:10 dilution, followed by transferring 1 ml of this mixture to the next tube with 9 ml of water for a 1:100 dilution, and so on. The script also mentions that some assays require up to seven steps of dilution, while others might use a twofold dilution method. The video concludes with a call to action for viewers to subscribe, follow on Twitter, and engage with the content.
Mindmap
Keywords
💡Serial Dilution
💡Dilution Factor
💡Stock Solution
💡Diluents
💡10-Fold Dilution
💡Tubes
💡Mixing
💡Transferring
💡Bacterial Concentrations
💡Twofold Dilution
💡Resuspending
Highlights
Serial dilution is a stepwise dilution by a constant dilution factor.
Most common dilution factors are 10-fold or 2-fold.
A 10-fold dilution means each step contains 10 times less of the content than the previous one.
The choice of dilution medium, such as water or a specific solution, is crucial for the serial dilution process.
For a 10-fold serial dilution, the initial step involves filling tubes to 9/10 of their capacity.
Adding 1 ml of stock solution to 9 ml of water achieves a 1:10 dilution.
Proper mixing is essential after each dilution step.
Transferring 1 ml from one dilution to the next with additional water continues the serial dilution process.
The final concentration after each transfer is determined by the dilution factor and the volume of water added.
Serial dilutions can be extended with careful resuspension if necessary.
Some assays for determining bacterial concentrations involve up to seven steps of 10-fold dilutions.
Twofold serial dilutions involve equal volumes of medium and stock solution for each step.
The process of twofold dilution is repeated by transferring equal volumes of the mixture to subsequent tubes.
The video demonstrates the practical application of serial dilutions in lab techniques and essays.
The channel encourages viewers to subscribe and turn on notifications for more content.
Support from viewers has contributed to the channel's growth.
The presenter thanks the audience for their support and encourages engagement through likes and comments.
The presenter invites viewers to follow on Twitter for updates and interaction.
Transcripts
Browse More Related Video

How to Use the Dilution Equation
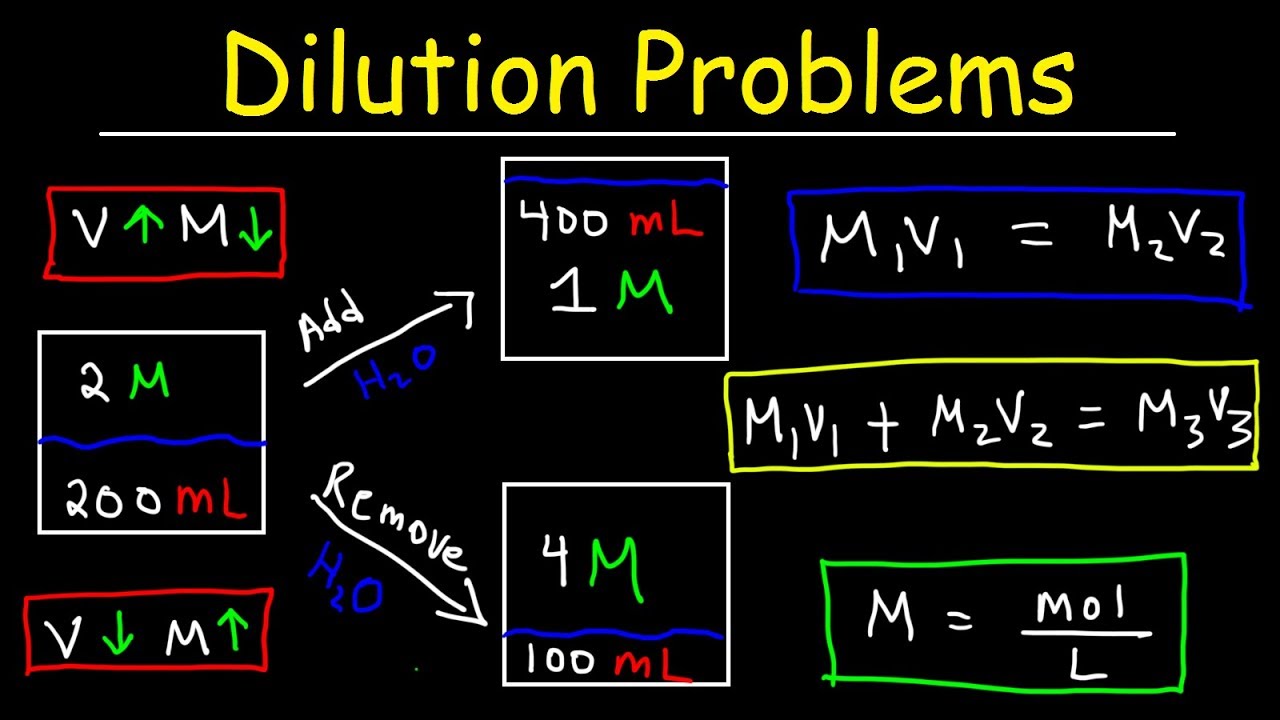
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
Dilution Explained

Practice Problem: Dilution Calculations

Concentration and Molarity: The Key to Chemical Solutions

Variation of conductivity with dilution- Part 1 | Electrochemistry | Chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: