Practice Problem: Dilution Calculations
TLDRThis educational video script covers dilution problems in chemistry, focusing on three specific questions. It explains the fundamental dilution equation, m1v1 = m2v2, where m represents molarity and v represents volume. The script guides viewers through calculating the concentration of a diluted sodium chloride solution, determining the volume of a diluted HCl solution, and finding the required volume of a stock NaOH solution to prepare a specific molarity. It encourages viewers to check a tutorial on solutions and dilution for further understanding and to try the problems themselves after gaining the necessary knowledge.
Takeaways
- 🧪 Dilution problems are discussed using the formula m1v1 = m2v2, where m represents molarity and v represents volume.
- 🔍 The first problem involves diluting a 0.850 L of 5.00 M NaCl solution to a final volume of 1.80 L, aiming to find the final concentration.
- 📐 The formula is rearranged to solve for the final concentration (m2) by dividing both sides by the final volume (v2).
- 🧩 The initial concentration (m1) is 5.00 M, and the initial volume (v1) is 0.850 L. The final volume (v2) after dilution is 1.80 L.
- ✅ The calculated final concentration (m2) for the first problem is 2.36 M, confirming that dilution results in a lower concentration.
- 🔄 The second problem asks for the volume of 0.12 M HCl that can be prepared from 11 mL of a 0.45 M stock solution.
- 📉 To find the volume of the diluted solution (v2), the formula is rearranged to divide m1v1 by m2.
- 📏 The stock solution is 0.45 M, and 11 mL of it is used. The desired final concentration (m2) is 0.12 M.
- 📐 The volume of the diluted solution (v2) is calculated to be 41 mL, which is larger than the stock solution volume, as expected.
- 🚰 The third problem requires determining the volume of 1.59 M NaOH needed to prepare a 5.00 L solution of 0.1 M NaOH.
- 🔄 For this, the formula is rearranged to solve for the volume of the stock solution (v1) by dividing m2v2 by m1.
- 📘 The desired final concentration (m2) is 0.1 M, and the final volume (v2) is 5.00 L. The stock solution concentration (m1) is 1.59 M.
- 📏 The volume of the stock solution (v1) required is calculated to be 0.314 L, which is significantly smaller than the final volume.
Q & A
What is the formula used for dilution problems?
-The formula used for dilution problems is m1v1 = m2v2, where m1 and m2 are the initial and final concentrations (molarity), and v1 and v2 are the initial and final volumes.
What is the initial concentration of the sodium chloride solution in the first problem?
-The initial concentration of the sodium chloride solution is 5.00 M (molar).
What volume was the sodium chloride solution diluted to in the first problem?
-The sodium chloride solution was diluted to a volume of 1.80 liters.
What is the final concentration of the diluted sodium chloride solution calculated in the first problem?
-The final concentration of the diluted sodium chloride solution is 2.36 M.
How many liters of stock solution of HCl were used in the second problem?
-Eleven milliliters of stock solution of HCl were used in the second problem.
What is the concentration of the stock solution of HCl in the second problem?
-The concentration of the stock solution of HCl is 0.45 M.
What volume of 0.12 M HCl can be prepared from the given stock solution in the second problem?
-You can prepare 41 milliliters of 0.12 M HCl from the given stock solution.
In the third problem, what is the desired final concentration of the NaOH solution?
-The desired final concentration of the NaOH solution is 0.1 M.
What is the concentration of the stock solution of NaOH in the third problem?
-The concentration of the stock solution of NaOH is 1.59 M.
What volume of the stock solution of NaOH is required to prepare 5 liters of 0.1 M NaOH solution in the third problem?
-0.314 liters of the stock solution of NaOH is required to prepare 5 liters of 0.1 M NaOH solution.
How can you verify if the dilution calculation is correct?
-You can verify the dilution calculation by ensuring that the final concentration is lower than the initial concentration and that the final volume is greater than the initial volume used.
What tutorial is recommended for those who find dilution problems confusing?
-The tutorial on solutions and dilution is recommended for those who need help understanding dilution calculations.
Outlines
🧪 Dilution Problems with Sodium Chloride Solution
The first paragraph introduces a chemistry tutorial focused on dilution problems. It presents a problem involving the dilution of a 0.850-liter, 5.00 molar sodium chloride solution to a final volume of 1.80 liters with water. The goal is to find the concentration of the diluted solution. The video suggests using the dilution equation m1v1 = m2v2, where m1 and v1 are the initial molarity and volume, and m2 and v2 are the final molarity and volume. By rearranging the equation to solve for m2, the initial concentration (5 molar), initial volume (0.850 liters), and final volume (1.80 liters) are substituted into the equation, yielding a final concentration of 2.36 molar. The explanation emphasizes the logical check that a more concentrated initial solution results in a less concentrated final solution after dilution.
📚 Preparing HCl and NaOH Solutions from Stock Solutions
The second paragraph continues the tutorial with two additional dilution problems. The first problem asks for the volume of 0.12 molar HCl that can be prepared from 11 milliliters of a 0.45 molar stock solution. Using the dilution equation m1v1 = m2v2, the video demonstrates solving for v2, the volume of the diluted solution. By substituting the known values, the calculation results in a final volume of 41 milliliters for the diluted HCl solution. The explanation includes a logical check to ensure that the final volume is larger than the initial volume, which is consistent with the dilution process. The third problem involves determining the volume of a 1.59 molar NaOH stock solution needed to prepare a 5-liter solution of 0.1 molar NaOH. The process involves solving for v1, the volume of the stock solution required, by rearranging and applying the dilution equation. The calculation shows that 0.314 liters of the stock NaOH solution are needed to achieve the desired diluted solution. The video concludes with a logical check to confirm that the volume of the stock solution is significantly smaller than the final diluted solution volume.
Mindmap
Keywords
💡Dilution
💡Molarity
💡Sodium Chloride
💡Volume
💡Concentration
💡Hydrochloric Acid (HCl)
💡Stock Solution
💡NaOH
💡Tutorial
💡Equation m1v1 = m2v2
💡Calculations
Highlights
Introduction to solving dilution problems using the equation m1v1 = m2v2.
Explanation of how to calculate the concentration of a diluted solution.
Demonstration of solving the first dilution problem involving sodium chloride.
Use of the dilution formula to find the concentration after dilution.
Verification of the dilution result by comparing initial and final concentrations.
Transition to solving the second dilution problem with HCl stock solution.
Calculation of the volume of diluted HCl that can be prepared from a stock solution.
Explanation of how to determine the volume of the diluted solution (v2).
Verification of the calculated volume by ensuring it's larger than the stock solution.
Introduction to the third dilution problem involving NaOH stock solution.
Determination of the volume of stock solution required for a specific diluted solution.
Use of the dilution formula to solve for the volume of the stock solution (v1).
Calculation of the volume of 1.59 molar NaOH needed for a 0.1 molar solution.
Verification of the dilution by comparing the volume of stock solution to the final solution.
Emphasis on the importance of checking the logic of dilution calculations.
Encouragement to watch a tutorial on solutions and dilution for further understanding.
Transcripts
Browse More Related Video
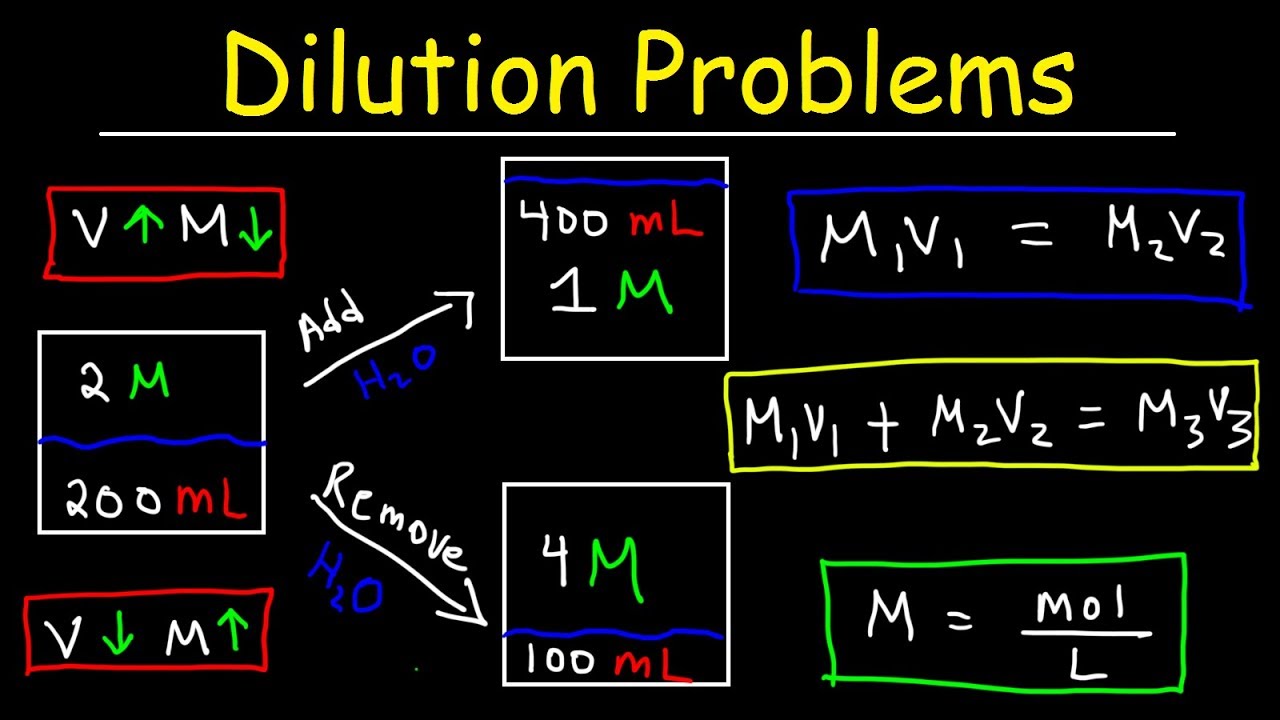
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations
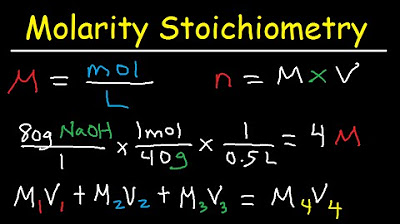
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry

How to Use the Dilution Equation

Molarity, Solution Stoichiometry and Dilution Problem
Dilution Explained

Concentration and Molarity: The Key to Chemical Solutions
5.0 / 5 (0 votes)
Thanks for rating: