How to Use the Dilution Equation
TLDRThe video script offers a detailed guide on how to dilute a concentrated stock solution to create a more dilute solution in a biology laboratory setting. It emphasizes the importance of using the dilution equation, which involves the concentration (c) and volume (v) of the solutions. The script provides step-by-step calculations using both percentage and milligrams per milliliter as units of concentration. It demonstrates how to manipulate the dilution equation to find the required volume of the concentrated solution needed to achieve the desired dilution. The example calculations show how to prepare a 5% solution from a 10% stock solution and a 10 micrograms per milliliter solution from a 10 milligrams per milliliter stock solution. The process highlights the use of basic metric conversions and the necessity of ensuring that the final volume of the diluted solution meets the intended volume, which in the examples provided is 100 milliliters.
Takeaways
- 🧪 Diluting a concentrated stock solution to a desired concentration involves adding more solvent to the solution.
- 📐 The dilution equation is a fundamental tool in calculating the required volumes of the stock solution and the solvent for a desired dilution.
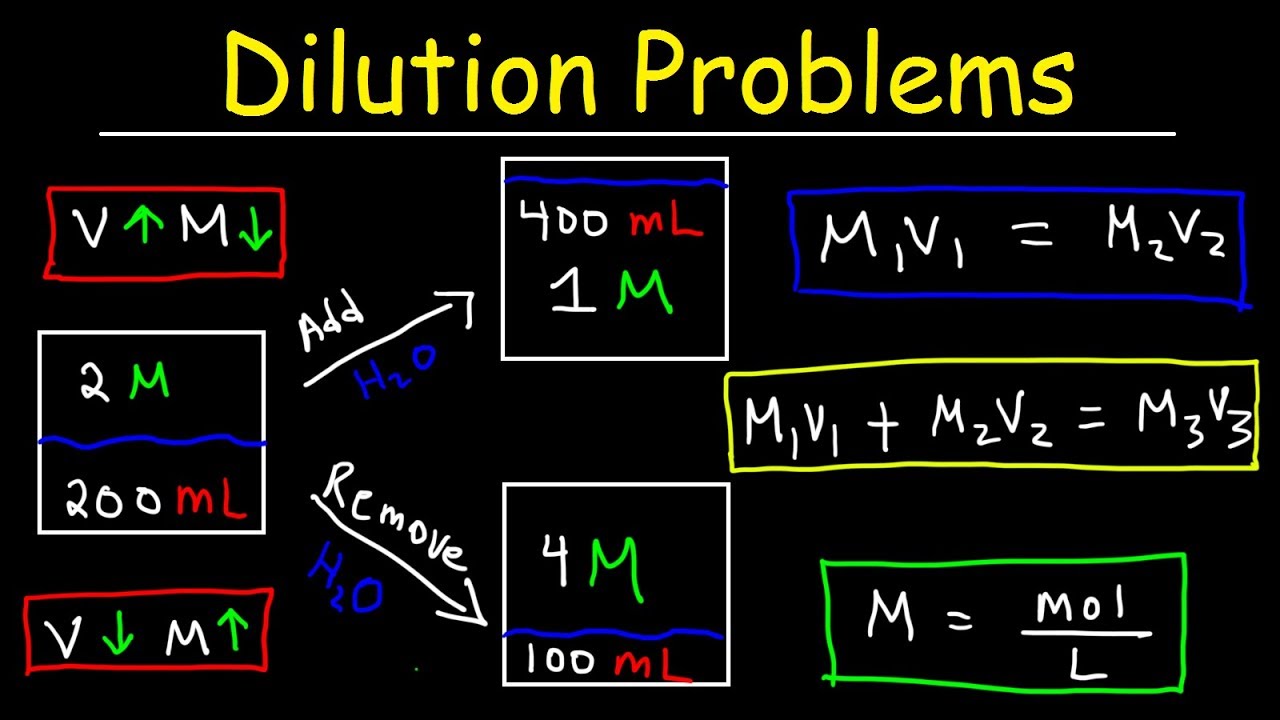
- 🔍 The dilution equation is given as c1v1 = c2v2, where c1 and v1 are the concentration and volume of the stock solution, and c2 and v2 are the desired concentration and volume of the dilute solution.
- 💡 To find the volume of the stock solution needed (v1), you can rearrange the dilution equation to solve for v1.
- 📉 If you want to decrease the concentration of a solution, you add more solvent to it.
- 📚 An example given in the script is to dilute a 10% stock solution to a 5% solution, which is achieved by adding solvent to half the volume of the stock solution.
- 🏥 In a biology lab, you may be given a solution in milligrams per milliliter (mg/mL), and you need to convert units to use the dilution equation correctly.
- ⚖️ Unit conversion is crucial when using the dilution equation with different units, such as converting milligrams to micrograms.
- 🔢 For small volumes, like microliters, it's important to use precise tools to measure the correct amount of stock solution and solvent.
- 🧊 When diluting, ensure the final volume of the solution equals the desired volume, which may require calculating the amount of solvent to add to the stock solution.
- 🔬 The script provides a step-by-step example of how to calculate the dilution of a 10 mg/mL stock solution to a 10 µg/mL solution to make 10 mL of the final solution.
- 📦 The final step in the dilution process is to add the calculated volume of the stock solution to the calculated volume of solvent to achieve the desired concentration and volume.
Q & A
What is the main topic of the video?
-The main topic of the video is how to calculate and prepare a dilute solution from a more concentrated stock solution in a biology laboratory.
What is the difference between solution one and solution two in terms of concentration?
-Solution one is more concentrated than solution two. The difference is in the amount of solute present in each of the solutions, with solution one being darker and having more solute.
What is the dilution equation used in the video?
-The dilution equation used in the video is C1V1 = C2V2, where C1 and V1 are the concentration and volume of the original solution, and C2 and V2 are the concentration and volume of the desired diluted solution.
How does the video demonstrate the process of diluting a 10% solution to a 5% solution?
-The video uses the dilution equation to show that if you want to make 100 milliliters of a 5% solution from a 10% solution, you would need to use 50 milliliters of the 10% solution and add 50 milliliters of water.
What is the key to solving for the volume of the original solution (V1) in the dilution equation?
-The key to solving for V1 is to set up the dilution equation with the known concentrations and volumes and then perform algebraic manipulation to isolate V1, solving for the required volume of the original solution.
How does the video handle different units of concentration in the dilution calculation?
-The video demonstrates converting different units of concentration (e.g., milligrams per milliliter to micrograms per milliliter) to ensure that both the numerator and denominator of the dilution equation have the same units before performing the calculation.
What is the example calculation provided in the video for diluting a solution?
-The example calculation involves diluting a solution with a concentration of 10 milligrams per milliliter to a concentration of 10 micrograms per milliliter, with the goal of making 10 milliliters of the diluted solution.
What is the final volume of the diluted solution in the example calculation?
-The final volume of the diluted solution in the example calculation is 10 milliliters.
How much of the original solution is needed to make the diluted solution in the example calculation?
-In the example calculation, 10 microliters of the original 10 milligrams per milliliter solution is needed to make 10 milliliters of a 10 micrograms per milliliter solution.
What is the total amount of water needed to be added to the original solution to achieve the final volume in the example calculation?
-To achieve the final volume of 10 milliliters in the example calculation, 9990 microliters of water need to be added to the 10 microliters of the original solution.
What tools are typically used to measure small volumes like microliters in a laboratory?
-Tools typically used to measure small volumes like microliters in a laboratory include micropipettes and pipette controllers, which allow for precise measurement and transfer of liquids.
Why is it important to match the units of concentration in the dilution equation before performing the calculation?
-Matching the units of concentration in the dilution equation is important to ensure the mathematical accuracy of the calculation. Different units of concentration cannot be directly compared or canceled out, so they must be converted to a common unit before proceeding with the calculation.
Outlines
🧪 Understanding Dilution in the Lab
This paragraph explains the process of diluting a concentrated stock solution to a more dilute solution in a biology lab. It emphasizes the difference in solute concentration between two solutions with the same volume. The key to dilution is using the dilution equation, which involves the concentration (c) and volume (v) of the solutions. The speaker illustrates how to use the equation with an example involving percentages and then with a more complex example using milligrams per milliliter. The goal is to calculate the volume of the concentrated solution (v1) needed to achieve a desired final concentration (c2) and volume (v2).
📐 Applying the Dilution Equation
The second paragraph delves into a practical example of using the dilution equation. It starts with an initial concentration (c1) of 10 milligrams per milliliter and a goal to create a solution (solution two) with a concentration (c2) of 10 micrograms per milliliter in a volume (v2) of 10 milliliters. The speaker demonstrates the mathematical steps to solve for the unknown volume of the initial solution (v1) required for the dilution. The process involves converting units to ensure consistency and using the dilution equation to find the necessary volume of the concentrated solution and water to achieve the desired dilution.
🚰 Preparing the Diluted Solution
The final paragraph focuses on the practical steps to prepare the diluted solution in the lab. It outlines the need to add a specific volume of water to the concentrated stock solution to achieve the desired final volume and concentration. The example provided involves adding 10 microliters of a 10 milligrams per milliliter stock solution to 9990 microliters of water to obtain a final 10-milliliter solution with a concentration of 10 micrograms per milliliter. The paragraph highlights the importance of precise measurement and the use of appropriate tools to handle small volumes, such as microliter pipettes.
Mindmap
Keywords
💡Dilute Solution
💡Stock Solution
💡Concentration
💡Volume
💡Dilution Equation
💡Solute
💡Solvent
💡Milliliters (mL)
💡Milligrams (mg)
💡Micrograms (µg)
💡Metric Conversion
Highlights
The video demonstrates how to calculate and prepare a dilute solution from a more concentrated stock solution in a biology laboratory.
When a solution is more concentrated than needed, dilution is necessary to achieve the desired concentration for laboratory use.
The difference between two solutions with the same volume but different color intensity is the amount of solute present.
The dilution equation, using variables c (concentration) and v (volume), is introduced to calculate the required dilution.
The formula c1v1 = c2v2 is used, where c1 and v1 are the concentration and volume of the initial solution, and c2 and v2 are the desired values.
A mathematical manipulation of the dilution equation is shown to find the volume of the initial solution (v1) needed to achieve the desired concentration (c2).
An example calculation is provided using a 10% concentrated solution to prepare a 5% solution, resulting in a need for 50 milliliters of the initial solution.
By adding water to the initial solution, the concentration is halved, achieving the goal of going from 10% to 5% concentration.
In laboratory practice, solutions are often measured in milligrams per milliliter rather than percentages.
Background research is recommended to determine the appropriate dilution concentration for specific applications, such as an antibiotic solution.
A second example calculation is presented, converting between milligrams per milliliter and micrograms per milliliter.
The calculation shows that 10 microliters of a 10 mg/mL solution are needed to make 10 mL of a 10 µg/mL solution.
The importance of using the correct tools to measure small volumes, such as microliters, is emphasized for precision in solution preparation.
The final step in the dilution process is to add the calculated volume of the stock solution to the required amount of water to reach the final desired volume.
The video provides a clear understanding of how to perform dilutions in a biology lab, which is crucial for accurate and safe chemical handling.
Metric conversions play a key role in dilution calculations, particularly when converting between different units of concentration.
The video concludes with a practical application example of preparing a 10 µg/mL solution from a 10 mg/mL stock solution using microliter measurements.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: