Dilution Explained
TLDRIn this educational video, Mr. Millington introduces the concept of dilution, which is the process of adding more solvent to a solution to decrease its concentration. He uses the example of diluting highly concentrated hydrochloric acid for school use, explaining the importance of safety and the practicality of making a large amount of diluted solution from a smaller volume of concentrated acid. The video then delves into the dilution formula (m1*v1 = m2*v2), demonstrating how to calculate the volumes needed for various dilution problems. Mr. Millington works through several examples, including making a 0.5 liter 1 molar solution from 12 molar HCl, determining the volume of sulfuric acid needed for a specific dilution, and calculating the amount of water required to achieve a desired solution concentration. The presentation aims to clarify the dilution process and its mathematical calculations for students.
Takeaways
- 🧪 Dilution is the process of adding more solvent to a solution, resulting in a less concentrated mixture.
- 🏫 An example of dilution is using 1.5 liters of super concentrated hydrochloric acid for an entire school year by diluting it with water.
- ⚠️ Safety first: When diluting, add acid to water, not water to acid, to prevent dangerous reactions.
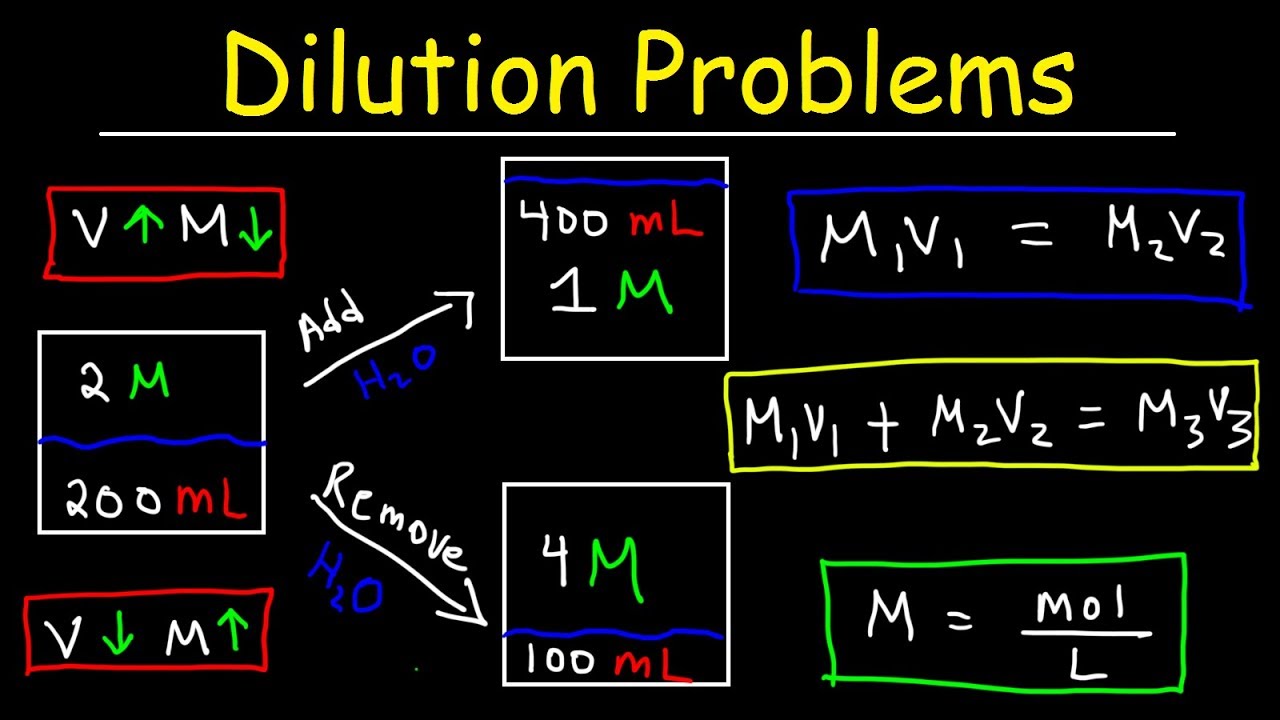
- 📚 The dilution formula is M1 * V1 = M2 * V2, where M represents molarity (moles per liter) and V represents volume (liters).
- 🔍 To find the volume of concentrated solution needed for a dilution, use the rearranged formula V1 = (M2 * V2) / M1.
- 📉 The more concentrated solution is represented by M1 and V1, while the diluted solution is represented by M2 and V2.
- 📝 When solving word problems, identify the desired final volume (V2) and molarity (M2) to calculate the required volume of the concentrated solution (V1).
- 💧 To calculate the amount of water needed for dilution, subtract the volume of the concentrated solution from the final desired volume.
- 📉 The concentration of the final solution can be calculated if the volume and molarity of the diluted solution are known.
- 🔢 Example calculations in the script include determining the volume of concentrated sulfuric acid needed to make a specific volume and molarity of a diluted solution.
- 📚 The script provides a comprehensive guide on how to apply the dilution formula to various chemistry problems involving the preparation of solutions with specific concentrations.
Q & A
What is dilution?
-Dilution is the process of adding more solvent to a solution, which results in the solution becoming less concentrated and more diluted.
What is an example of a highly concentrated solution used in the script?
-An example of a highly concentrated solution mentioned in the script is 12 molar hydrochloric acid, which is one of the strongest forms of hydrochloric acid available.
Why is it necessary to dilute the 12 molar hydrochloric acid?
-The 12 molar hydrochloric acid is diluted to prevent severe damage in case it comes into contact with skin or eyes, making it safer for use by students.
What is the dilution formula and how is it used?
-The dilution formula is M1V1 = M2V2, where M1 and V1 represent the molarity and volume of the concentrated solution, and M2 and V2 represent the molarity and volume of the diluted solution. It is used to calculate the volumes needed to achieve a desired molarity.
How much of the 12 molar sulfuric acid is needed to make 1.500 liters of a 1.500 molar solution?
-To make 1.500 liters of a 1.500 molar solution, 0.1875 liters of the 12 molar sulfuric acid is needed.
What is the relationship between the volume of concentrated solution and the volume of water needed to dilute it?
-The volume of water needed to dilute the concentrated solution can be found by subtracting the volume of the concentrated solution from the total desired volume of the diluted solution.
How much water must be added to make 2.5 liters of a 2 molar solution of hydrochloric acid from 16 molar hydrochloric acid?
-To make 2.5 liters of a 2 molar solution from 16 molar hydrochloric acid, 2.187 liters of water must be added.
What is the concentration of the nitric acid used if 1.75 liters of it is used to make 5 liters of 1.2 molar nitric acid?
-The concentration of the nitric acid used was 3.49 molar.
Why is it important to add acid to water and not water to acid when diluting?
-It is important to add acid to water and not water to acid to prevent a violent reaction and to control the dilution process safely.
How can you determine the molarity of a stock solution if you know the volume and molarity of the diluted solution?
-You can determine the molarity of a stock solution by using the dilution formula M1V1 = M2V2, where M1 and V1 are the unknowns, and solving for M1 after knowing M2 (molarity of the diluted solution) and V2 (volume of the diluted solution).
Outlines
🧪 Understanding Dilution and Its Formula
In this segment, Mr. Millington introduces the concept of dilution, which is the process of adding more solvent to a solution to decrease its concentration. He uses the example of diluting super concentrated hydrochloric acid to a safer, one molar solution for educational purposes. The dilution formula, m_1 × v_1 = m_2 × v_2, is explained as a tool to calculate the volumes needed for creating a dilute solution from a concentrated one. The importance of safety when handling concentrated acids is highlighted, and the process of dilution is simplified through the formula, making it accessible for solving various dilution-related problems.
📚 Applying the Dilution Formula to Solve Problems
This paragraph demonstrates the application of the dilution formula to solve specific problems. The first example involves calculating the volume of 12 molar sulfuric acid needed to create 1.5 liters of a 1.5 molar solution, resulting in the need for 0.1875 liters of the concentrated acid. The subsequent discussion explores how much water would be required to achieve the desired dilution. Another example calculates the amount of water needed to dilute 16 molar hydrochloric acid to a 2 molar solution in 2.5 liters, revealing the necessity for 2.187 liters of water. The explanation is clear and methodical, guiding the audience through the calculations step by step.
🧐 Determining the Concentration of Diluted Solutions
The final paragraph focuses on determining the original concentration of a solution given the volume and concentration of the diluted solution. Using the dilution formula in reverse, Mr. Millington shows how to calculate the molarity of the stock solution of nitric acid, which was used to make 5 liters of a 1.2 molar solution starting with 1.75 liters. The calculation results in the original molarity of the nitric acid being 3.49 molar. This example illustrates a practical application of the dilution formula to backtrack from a dilute solution to determine the concentration of the concentrated solution used.
Mindmap
Keywords
💡Dilution
💡Solvent
💡Concentration
💡Molarity
💡Hydrochloric Acid
💡Dilution Formula
💡Volume
💡Example Problems
💡Safety
💡Lab Preparation
Highlights
Introduction to the concept of dilution as the process of adding more solvent to a solution.
Explanation of how dilution reduces the concentration of a solution.
Example of using super concentrated hydrochloric acid in a school setting.
Dilution of strong hydrochloric acid to prevent harm to students.
Demonstration of how to dilute 1.5 liters of concentrated hydrochloric acid to make a 0.5-liter 1M solution.
Introduction to the dilution formula (m1*v1 = m2*v2).
Application of the dilution formula to solve various dilution problems.
Clarification on the terms molarity (m) and volume (v) in the dilution formula.
Guidance on identifying the more concentrated (m1, v1) and more diluted (m2, v2) solutions in word problems.
Problem-solving example: Calculating the volume of 12M sulfuric acid needed for a 1.5-liter 1.5M solution.
Method to determine the amount of water needed to dilute a solution.
Example problem: Calculating water addition for a 2.5-liter 2M hydrochloric acid solution from 16M stock.
Explanation of how to find the volume of concentrated acid needed using the dilution formula.
Calculation of water volume required to reach the final solution volume.
Example problem: Determining the concentration of nitric acid used to make a 5-liter 1.2M solution.
Process of finding the initial molarity (m1) of a solution using the given volumes and final molarity.
Conclusion summarizing the concept of dilution and the application of the dilution formula.
Transcripts
Browse More Related Video

Concentration and Molarity: The Key to Chemical Solutions

Molarity, Solution Stoichiometry and Dilution Problem

How to Use the Dilution Equation

Practice Problem: Dilution Calculations
Dilution Problems, Chemistry, Molarity & Concentration Examples, Formula & Equations

11.2 Dilutions and Solution Stoichiometry | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: