Concentration and Molarity: The Key to Chemical Solutions
TLDRIn this chemistry lesson, Mr. Cozzi introduces the concepts of concentration, molarity, and dilution. He explains that solutions are homogeneous mixtures of solute and solvent, with water being the most common solvent. Molarity is defined as moles of solute per liter of solution, providing a quantitative measure for solution concentration. The lesson includes practical examples, such as calculating the molarity of an ammonium nitrate solution and preparing a diluted sulfuric acid solution from a concentrated one. Mr. Cozzi emphasizes the importance of practice in mastering the conversion between grams to moles and understanding the relationship between molarity and volume during dilution.
Takeaways
- 🧪 The lesson is about understanding concentration and molarity in chemistry.
- 📚 Students need a periodic table and a calculator for the lesson.
- 🌐 Solutions are defined as homogeneous mixtures of solute and solvent, with water often being the solvent.
- 🔍 Concentration is the ratio of the amount of solute to the amount of solution or solvent.
- 📉 Adding more solute increases concentration, while adding more solvent decreases it.
- 🧪 Molarity is a quantitative term representing moles of solute per liter of solution.
- 📉 An example is given to calculate molarity, involving converting grams to moles and milliliters to liters.
- 🔄 Dilution is the process of increasing the amount of solvent to decrease the concentration of a solution.
- ⚖️ To prepare dilutions, an equation relating the molarity and volume of the initial and final solutions is used.
- 📝 An example problem demonstrates how to prepare a diluted solution of sulfuric acid from a concentrated stock solution.
- 📚 The importance of practice in converting grams to moles and calculating molarity is emphasized.
Q & A
What is the main topic of the chemistry lesson presented by Mr. Cozzi?
-The main topic of the chemistry lesson is concentration and molarity, including understanding solutions, dilution, and how to calculate molarity.
What are the tools Mr. Cozzi suggests students should have ready for the lesson?
-Mr. Cozzi suggests students should have a periodic table and a calculator with fresh batteries ready for the lesson.
What is a solution according to Mr. Cozzi's explanation?
-A solution is a homogeneous mixture that consists of a solute, which is the substance being dissolved, and a solvent, which does the dissolving, often water.
What is the significance of water being referred to as the 'universal solvent'?
-Water is called the 'universal solvent' because it dissolves more substances than any other liquid, making it very important in chemistry.
How is concentration defined in the context of the lesson?
-Concentration is defined as the ratio of the amount of solute to the amount of solution or solvent, essentially solute per solution.
What is molarity and how is it represented?
-Molarity is a quantitative term that represents the moles of solute per liter of solution, and it is represented by the capital letter M.
Can you provide an example of how to calculate molarity as shown in the lesson?
-Sure. To calculate the molarity of a solution prepared by dissolving 16.75 grams of ammonium nitrate in water to make 275 moles of solution, first find the molar mass of ammonium nitrate, then convert grams to moles, and finally divide the moles by the volume in liters to get molarity.
What is the concept of dilution in the context of this chemistry lesson?
-Dilution is the process of increasing the amount of solvent in a solution, which decreases the concentration of the solute.
How can one prepare a dilution of a stock solution according to the lesson?
-To prepare a dilution, you take a stock solution of high molar concentration and add solvent to it. You use the equation M1V1 = M2V2, where M1 and V1 are the molarity and volume of the stock solution, and M2 and V2 are the desired molarity and volume of the dilution.
What is the importance of practice when learning about molarity and dilution?
-Practice is important because it helps students become proficient in converting grams to moles and understanding how to divide moles by the volume of the solution to calculate molarity.
How can students get in touch with Mr. Cozzi if they have questions about the lesson?
-Students can send an email to Mr. Cozzi at mr.cozzi@mrcozzi.com for any questions they might have.
Outlines
🧪 Chemistry of Concentration and Molarity
Mr. Cozzi introduces a chemistry lesson focusing on concentration and molarity. He outlines the importance of understanding solutions, solute, solvent, and the concepts of concentration and dilution. The lesson emphasizes the quantitative measurement of molarity, which is moles of solute per liter of solution. An example calculation is provided to demonstrate how to find the molarity of a solution made by dissolving ammonium nitrate in water. The process involves converting grams to moles and milliliters to liters, and using the molar mass from the periodic table to calculate the moles of solute.
📚 Making Dilutions and Understanding Molarity
This paragraph delves into the process of making dilutions from stock solutions with high molar concentrations. Mr. Cozzi explains the concept of molarity and how it changes when solvent is added, while the number of moles of solute remains constant. He introduces an algebraic equation that relates the molarity and volume of the initial and final solutions, allowing for the calculation of dilutions. An example is given to illustrate the preparation of a diluted sulfuric acid solution from a concentrated stock. The summary includes a step-by-step guide on using the equation to find the required volume of water to add to achieve the desired molarity.
👋 Closing Remarks and Additional Resources
The final paragraph serves as a closing note to the lesson. Mr. Cozzi encourages practice in converting grams to moles and understanding molarity calculations. He invites viewers to reach out with any questions via email and promotes his online resources, including PowerPoint videos and YouTube channels, for further learning and clarification on the topics covered in the lesson.
Mindmap
Keywords
💡Concentration
💡Molarity
💡Dilution
💡Solution
💡Solute
💡Solvent
💡Avogadro's Number
💡Molar Mass
💡Dilution Equation
💡Stock Solution
Highlights
Introduction to the lesson on concentration and molarity, emphasizing the importance of these concepts in chemistry.
Explanation of what students will learn: concentration, molarity, dilution, and how to make dilutions.
Mention of necessary materials: periodic table, calculator, knowledge of Avogadro's number, the mole, and molar mass.
Definition and explanation of solutions, including the concepts of solute and solvent.
Discussion of concentration as the ratio of solute to solution or solvent, and how adding more solute or solvent affects concentration.
Introduction to dilution and how increasing the amount of solvent decreases concentration.
Transition to the need for quantitative terms in science, leading to the introduction of molarity.
Definition of molarity: moles of solute per liter of solution, and its significance for quantitative calculations.
Step-by-step example calculation of molarity using ammonium nitrate, including conversions from grams to moles and milliliters to liters.
Explanation of the molar mass calculation for ammonium nitrate using the periodic table.
Detail on calculating the moles of ammonium nitrate and using it to determine the molarity of the solution.
Introduction to the concept of dilution using a stock solution and the mathematical relationship between initial and final concentrations and volumes.
Example problem demonstrating how to prepare a diluted solution from a concentrated stock solution using the M1V1 = M2V2 formula.
Recap of the key points covered: solutions, concentration, dilution, molarity, and practical examples.
Emphasis on the importance of practice in mastering molarity calculations, along with encouragement to use provided resources and ask questions.
Transcripts
Browse More Related Video

Concentration and Molarity explained: what is it, how is it used + practice problems

Molarity versus Molality
What is molarity and molality Class 11? | What is molality and example? | calculate molality
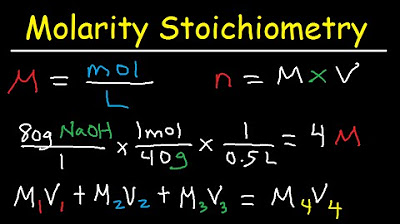
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry

How to Calculate Molality

Difference between Molarity and Molality
5.0 / 5 (0 votes)
Thanks for rating: