Understanding Molecular & Formula Mass in Chemistry
TLDRThis lesson delves into the concept of atomic mass units and how they are used to calculate the mass of molecules and ionic compounds. By utilizing the periodic table, the video explains the calculation of molecular mass for covalent compounds and formula mass for ionic compounds, emphasizing the importance of these calculations in understanding chemical reactions. The lesson provides examples, including calculating the mass of common substances like water, carbon dioxide, and glucose, as well as more complex compounds like nitric acid and potassium permanganate, to solidify the concept.
Takeaways
- 📚 The goal of chemistry is to calculate and determine the outcomes of chemical reactions, not just discuss them theoretically.
- 🔍 Understanding atomic mass is fundamental in chemistry, as it measures the mass of individual atoms and helps in calculating the mass of compounds.
- 📈 The periodic table provides atomic masses, which are the weighted averages of all isotopes of an element found in nature.
- 🌟 Atomic mass units (AMU) are defined based on the mass of carbon-12, where 12 AMU is exactly equal to the mass of one atom of carbon-12.
- 💡 The mass of a molecule is referred to as its molecular mass, while the mass of an ionic compound is called its formula mass.
- 🧪 When calculating molecular or formula mass, you multiply the atomic mass of each element by the number of its atoms in the compound and sum these values.
- 🔬 Isotopes of an element differ in mass due to varying numbers of neutrons, and the atomic mass listed on the periodic table accounts for these differences.
- 📊 In chemical notation, the subscript numbers indicate the number of atoms of each element in a compound, which is crucial for calculating mass.
- 🌐 The concept of molecular and formula mass is universally applicable, allowing for the comparison of masses of different compounds.
- 🚀 Practice is essential for becoming proficient in calculating masses, which is a fundamental skill in problem-solving within chemistry.
Q & A
What is the primary goal of chemistry in relation to calculations?
-The primary goal of chemistry in relation to calculations is to be able to determine and predict the outcomes of chemical reactions by putting numbers on them, allowing for the calculation of things like the mass of products formed in a reaction.
How is atomic mass related to the mass of an atom?
-Atomic mass is a measure of how massive each atom is, based largely on the number of protons and neutrons in the nucleus since electrons have much less mass. It is expressed in atomic mass units (AMU), where 12 AMU is defined as the mass of one carbon-12 atom.
What is an atomic mass unit (AMU) defined in terms of?
-An atomic mass unit (AMU) is defined in terms of the carbon-12 isotope. Specifically, 12 AMU is exactly equal to the mass of one atom of carbon-12, which has six protons and six neutrons.
Why do the atomic masses on the periodic table not always match the defined atomic mass units?
-The atomic masses on the periodic table are not exact matches to the defined atomic mass units because they represent the weighted average of all the isotopes of an element that exist in nature, taking into account their relative abundances.
What is the difference between atomic mass and molecular mass?
-Atomic mass refers to the mass of a single atom, while molecular mass refers to the mass of a molecule, which is a collection of atoms bonded together. Atomic mass is used for individual atoms, and molecular mass is used for compounds formed by covalent bonding.
How do you calculate the molecular mass of a compound like water (H2O)?
-To calculate the molecular mass of a compound like water, you sum the atomic masses of all the atoms in the molecule. For water, this would be 2 times the atomic mass of hydrogen (approximately 1.01 AMU each) plus the atomic mass of oxygen (approximately 16.00 AMU), resulting in a molecular mass of about 18.02 AMU.
What is the term used for the mass of an ionic compound?
-The term used for the mass of an ionic compound is formula mass. This is because ionic compounds consist of a metal and a non-metal (or a polyatomic ion) that form a three-dimensional crystal lattice structure, not a single molecule.
How is the formula mass of an ionic compound like sodium chloride (NaCl) calculated?
-The formula mass of an ionic compound like sodium chloride is calculated by adding the atomic mass of the metal (sodium in this case, with an atomic mass of approximately 22.99 AMU) to the atomic mass of the non-metal (chlorine, with an atomic mass of approximately 35.45 AMU), resulting in a formula mass of approximately 58.44 AMU.
What is the significance of calculating formula masses and molecular masses in chemistry?
-Calculating formula masses and molecular masses is significant in chemistry because it is the first step in solving chemical reactions. Understanding the mass of reactants and products allows chemists to balance chemical equations and predict the outcomes of reactions.
How does the mass of a compound compare to the atomic mass units of its constituent elements?
-The mass of a compound, whether expressed as a formula mass or molecular mass, is a sum of the atomic masses of its constituent elements, taking into account the number of each type of atom present in the compound. This total mass is much larger than the atomic mass units of the individual elements, as it reflects the combined mass of all atoms in the compound.
What is the importance of understanding the difference between atomic mass, molecular mass, and formula mass in chemistry?
-Understanding the difference between atomic mass, molecular mass, and formula mass is crucial in chemistry as it allows for accurate calculations of reaction outcomes, proper balancing of chemical equations, and the ability to predict the behavior and properties of substances. It also helps in distinguishing between covalent compounds (molecular mass) and ionic compounds (formula mass).
Outlines
📚 Introduction to Atomic and Molecular Mass
This paragraph introduces the concept of atomic and molecular mass, explaining the objectives of the lesson. It emphasizes understanding the formation of molecules and ionic compounds, calculating their mass using the periodic table, and the importance of these calculations in chemistry. The lesson aims to transition from a general discussion to more mathematical approaches, highlighting the goal of chemistry to calculate and determine outcomes of chemical reactions.
🔍 Understanding Atomic Mass and Mass Units
The paragraph delves into the concept of atomic mass, which is the measurement of an atom's mass. It explains that atomic mass increases across the periodic table due to the increase in protons and neutrons. The electrons, while charged, contribute minimally to the mass. The paragraph introduces the concept of atomic mass units (AMU), defined based on the carbon-12 isotope, where 12 AMU is exactly equal to the mass of one carbon-12 atom. It also discusses the variability in the number of decimal places in different periodic tables and how isotopes affect the atomic mass values presented.
🧪 Calculation of Molecular Mass
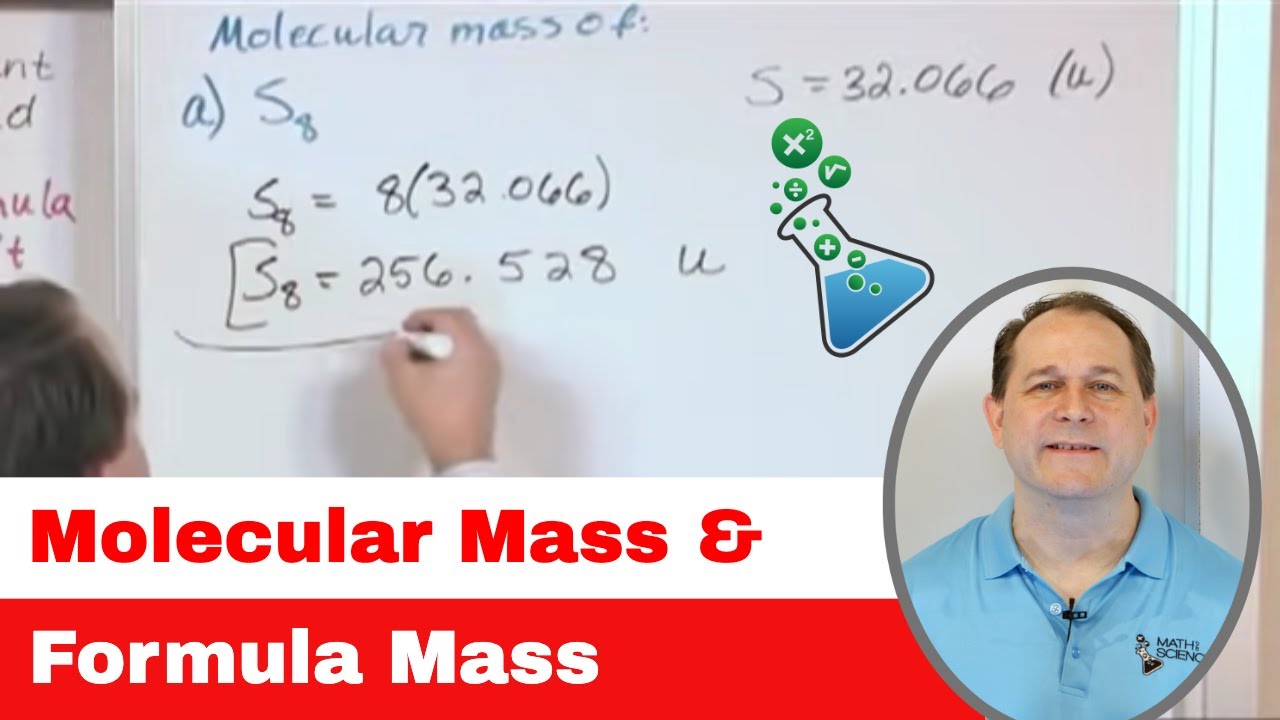
This section focuses on calculating molecular mass, differentiating it from atomic mass. It explains that while atomic mass refers to individual atoms, molecular mass refers to molecules composed of multiple atoms bonded together. The calculation involves summing the atomic masses of all atoms in the molecule, using the periodic table for the necessary values. The example of water (H2O) is used to illustrate the process, resulting in a molecular mass of 18.02 AMU.
📈 Formula Mass of Ionic Compounds
The paragraph discusses the concept of formula mass, which is used for ionic compounds. Unlike molecules, ionic compounds form a three-dimensional crystal lattice structure and do not behave as free-floating entities. The calculation of formula mass involves adding the atomic masses of the constituent metal and non-metal (or polyatomic ion) elements. Sodium chloride (NaCl) is used as an example, with its formula mass calculated as 58.44 AMU.
🌟 Practice with Molecular and Formula Mass Calculations
This part of the script emphasizes the importance of practice in calculating molecular and formula masses. It explains that repetition helps in becoming familiar with the atomic masses of common elements and the process of naming compounds. The paragraph provides examples of calculating the masses of nitrogen dioxide (NO2), butane (C4H10), and glucose (C6H12O6), highlighting the process and the resulting atomic mass units.
🧬 Calculation of Mass for Polyatomic Ions and Acids
The paragraph covers the calculation of mass for polyatomic ions and acids. It explains the process of identifying the constituent elements and their respective atomic masses, then summing them to find the formula mass. Examples include calculating the mass of chromium nitrate (Cr(NO3)3) and nitric acid (HNO3), with a focus on handling polyatomic ions like the nitrate ion (NO3-). The importance of memorizing certain compounds and using the periodic table effectively is also stressed.
📚 Summary and Further Practice
The final paragraph summarizes the lesson's focus on calculating the mass of compounds, whether they are molecules, ionic compounds, or polyatomic ions. It reiterates the difference between molecular mass (for covalently bonded non-metals), formula mass (for ionic compounds), and the importance of using the periodic table for these calculations. The paragraph encourages further practice to solidify understanding and prepare for more complex chemical reactions in future lessons.
Mindmap
Keywords
💡Atomic Mass
💡Atomic Mass Unit (AMU)
💡Molecular Mass
💡Formula Mass
💡Isotopic Abundance
💡Periodic Table
💡Covalent Bond
💡Ionic Bond
💡Chemical Reaction
💡Stoichiometry
Highlights
Understanding the formation of molecules and ionic compounds and calculating their mass is a key objective of the lesson.
The mass of compounds can be calculated using the atomic numbers on the periodic table.
Atomic mass is a measure of an atom's mass and is primarily determined by protons and neutrons.
The concept of atomic mass unit (AMU) is defined based on the mass of carbon-12, where 12 AMU is exactly equal to the mass of one atom of carbon-12.
The atomic masses listed on the periodic table are weighted averages of all isotopes of an element found in nature.
Molecular mass refers to the mass of a molecule, while atomic mass refers to the mass of a single atom.
The mass of water (H2O) can be calculated by adding the atomic masses of two hydrogen atoms and one oxygen atom.
The term 'formula mass' is used for ionic compounds, which are composed of a metal and a non-metal or a metal and a polyatomic ion.
Sodium chloride (NaCl) is an example of an ionic compound with a formula mass calculated by adding the atomic masses of sodium and chlorine.
The mass of a compound can be used to determine the ratio of elements in the compound, such as the 1:1 ratio in sodium chloride.
The lesson emphasizes the importance of calculating molecular and formula masses as the first step in solving chemical reactions.
The mass of nitrogen dioxide (NO2) is calculated by adding the atomic masses of nitrogen and two oxygen atoms.
Butane (C4H10) is an example of a hydrocarbon molecule with a mass calculated by adding the atomic masses of carbon and hydrogen.
Glucose (C6H12O6) is a simple sugar with a mass calculated by adding the atomic masses of carbon, hydrogen, and oxygen.
Chromium nitrate (Cr(NO3)3) is an ionic compound with a formula mass calculated by adding the atomic masses of chromium, nitrogen, and oxygen.
Nitric acid (HNO3) is an acid with a mass calculated by adding the atomic masses of hydrogen, nitrogen, and three oxygen atoms.
Potassium permanganate (KMnO4) is an ionic compound with a formula mass calculated by adding the atomic masses of potassium, manganese, and four oxygen atoms.
The lesson concludes with a reminder to practice calculating molecular and formula masses for various compounds to build proficiency in chemistry.
Transcripts
Browse More Related Video
01 - Molecular Mass And Formula Mass - Learn the Formula Unit, Molecular Formula & Formula Mass
Chemical Formulas, Ionic & Covalent Bonds in Chemistry - [1-2-14]

Mole Concept Tips and Tricks

Mole Conversions Made Easy: How to Convert Between Grams and Moles

How To Calculate The Molar Mass of a Compound - Quick & Easy!

BTEC Applied Science: Unit 1 Chemistry Relative Mass
5.0 / 5 (0 votes)
Thanks for rating: