[H2 Chemistry] 2023 Topic 2 Chemical Bonding 2
TLDRThis chemistry lecture delves into covalent bonding, contrasting it with ionic and metallic bonding, emphasizing the importance of understanding atomic structure and orbital overlap. It explains sigma and pi bonds, bond strength, and the role of electronegativity in bond polarity. The lecture also covers the concept of bond energy, homolytic bond breakage, and introduces Lewis structures, including exceptions to the octet rule and coordinate bonding. It provides practical examples and exercises to illustrate the formation and representation of various covalent and ionic compounds, preparing students for more advanced chemistry topics.
Takeaways
- 🧪 Covalent bonding is a key focus in chemistry, with a more in-depth study compared to secondary school, requiring attention to orbital overlaps and bond types like sigma and pi bonds.
- 📚 The prerequisite for understanding covalent bonding is familiarity with atomic structure, specifically s and p orbitals, which are crucial for bond formation.
- 🔬 Sigma bonds are formed by head-on or end-on overlaps of orbitals, while pi bonds result from side-on overlaps, with pi bonds being weaker due to less direct overlap.
- 🔑 The sequence of bond formation is important, with sigma bonds forming first, followed by pi bonds, which is a concept not always emphasized but fundamental for understanding molecular structure.
- ⚖️ Bond energy, measured in kilojoules per mole, defines the average energy required to break a mole of covalent bonds homolytically, and is a measure of bond strength.
- 💡 The strength of covalent bonds depends on the degree of orbital overlap and how strongly the electrons are held by the nuclei, with shorter bonds generally being stronger.
- 📉 An exception to the typical bond strength pattern is the fluorine molecule (F2), which has a weaker bond compared to its short length due to repulsion between lone pairs of electrons.
- 🔍 The bond order, which is the number of covalent bonds between a pair of atoms, can indicate bond strength, with double and triple bonds being stronger than single bonds, but not proportionally so.
- 🌟 Polar covalent bonds, such as the carbon-oxygen bond in CO, are stronger than non-polar covalent bonds due to electrostatic attraction, a concept that will be further explored in advanced chemistry.
- 🚫 The octet rule, which states that atoms tend to form enough bonds to have eight electrons in their valence shell, has exceptions, especially with elements like beryllium and boron that form electron-deficient compounds.
- 📝 Dot and cross diagrams are essential for representing the valence electrons in molecules and are used to draw Lewis structures, which are crucial for understanding molecular geometry and reactivity.
Q & A
What is the main focus of Lecture 2 in the provided transcript?
-The main focus of Lecture 2 is covalent bonding, including an in-depth exploration of how it differs from what is taught in secondary school, and the importance of understanding atomic structure as a prerequisite.
What are the two types of bonds that can be formed through orbital overlap?
-The two types of bonds formed through orbital overlap are sigma bonds, which result from head-on or end-on overlaps, and pi bonds, which result from side-on overlaps.
Why is it important to understand the formation of sigma and pi bonds in covalent bonding?
-Understanding the formation of sigma and pi bonds is important because it helps explain the strength and nature of covalent bonds. Sigma bonds are generally stronger due to direct orbital overlap, while pi bonds, which form from side-on overlap, are weaker.
What is the significance of the order of bond formation in a double bond?
-In a double bond, a sigma bond must be formed before a pi bond can be formed. This sequence is significant because it dictates the stability and strength of the bond, with the sigma bond providing the initial stability required for the pi bond to form.
How does the strength of a covalent bond relate to the extent of orbital overlap?
-The strength of a covalent bond is directly related to the extent of orbital overlap. The better the overlap between the orbitals of two atoms, the stronger the bond, as the electrons are more effectively held by the nuclei of both atoms.
What is bond energy, and how is it related to the strength of a covalent bond?
-Bond energy is the average amount of energy required to break one mole of a covalent bond in a homolytic manner, resulting in the formation of radicals. The greater the bond energy, the stronger the covalent bond, as more energy is needed to break it.
Why is the bond energy of a fluorine-fluorine (F-F) bond weaker than expected for its short bond length?
-The F-F bond energy is weaker than expected because of the repulsion between the lone pairs of electrons on the p orbitals of the fluorine atoms. This repulsion leads to a weakening of the bond despite the short bond length.
What is the difference between homolytic and heterolytic bond breakage?
-Homolytic bond breakage involves the equal redistribution of electrons between the atoms, forming radicals, whereas heterolytic bond breakage results in one atom gaining both electrons, forming an anion, and the other losing an electron, forming a cation.
What is the role of electronegativity in the formation of polar covalent bonds?
-Electronegativity plays a crucial role in the formation of polar covalent bonds. The difference in electronegativity between two atoms causes the electron density to be drawn more towards the more electronegative atom, creating a dipole moment and strengthening the bond through electrostatic attraction.
Why do some molecules with odd numbers of valence electrons form radicals, and how does this affect their reactivity?
-Molecules with odd numbers of valence electrons form radicals because they cannot pair all their electrons to achieve a stable configuration. This results in unpaired electrons, making the molecules highly reactive as they tend to participate in chemical reactions to achieve stability.
Outlines
🧪 Covalent Bonding and Sigma vs. Pi Bonds
The lecture delves into covalent bonding, contrasting it with ionic and metallic bonding, and emphasizing its complexity. It focuses on the formation of sigma and pi bonds, which result from head-on and side-on orbital overlaps respectively. The sigma bond is highlighted as the first bond to form, followed by pi bonds. The lecture also explains the prerequisites for understanding covalent bonding, which include familiarity with atomic structure and orbitals. Examples of s and p orbital overlaps are provided, such as between two hydrogen atoms for a sigma bond or a sigma bond between hydrogen and fluorine. The importance of the order of bond formation is reiterated, with sigma bonds forming before pi bonds, which is a concept not always clear in secondary school curriculums.
🌟 Understanding Sigma and Pi Bonds' Strength and Formation
This section further explores the nature of sigma and pi bonds, discussing the orientation of p orbitals and their involvement in bond formation. It explains that the use of a p orbital for sigma bond formation precludes the formation of additional sigma bonds, leading to the formation of pi bonds instead. The lecture also touches on the relative strength of these bonds, with pi bonds being weaker due to less direct orbital overlap. The concept of bond energy is introduced, with an emphasis on homolytic bond breakage, where bond energy is defined as the energy required to break a mole of covalent bonds homolytically. The lecture provides examples of bond energy in hydrogen and mentions the exception of fluorine, which has a weak bond energy due to electron repulsion between lone pairs on the p orbitals.
🔬 Bond Energy, Bond Order, and Bond Strength
The lecture continues with a deeper look at bond energy, explaining how it is measured and the significance of homolytic bond breakage in defining bond energy. It also discusses bond strength in relation to the extent of orbital overlap and the electrostatic hold on electrons by the nuclei. The concept of bond order is introduced as a proxy for the number of covalent bonds between atoms, and the strength of multiple bonds is compared, with double and triple bonds being stronger than single bonds. The lecture also explains the increased strength of polar covalent bonds due to electrostatic attraction and mentions that bond length and strength are inversely related, with shorter bonds being stronger.
📚 Covalent Bonding Exceptions and Dot and Cross Diagrams
This part of the lecture addresses exceptions to the octet rule, particularly for elements like beryllium and boron, which form electron-deficient compounds like beryllium dichloride and boron trifluoride. It also discusses the stabilization of these compounds through various means, such as polymeric forms or dimeric structures involving dative covalent bonds. The lecture then transitions into the importance of dot and cross diagrams for representing covalent bonds, especially in covalent compounds, and introduces the concept of Lewis structures.
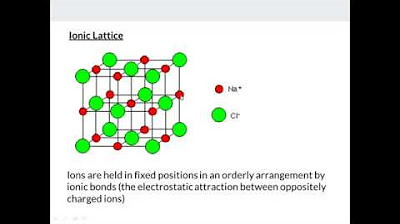
📝 Drawing Dot and Cross Diagrams for Ionic Compounds
The lecture provides a practical guide to drawing dot and cross diagrams for ionic compounds, using sodium chloride and magnesium oxide as examples. It explains the electron transfer process in ionic bonding and how to represent it in a dot and cross diagram, emphasizing the difference from secondary school teachings where valence electrons are typically shown. The lecture also covers the exercise on drawing dot and cross diagrams for various ionic compounds, highlighting the process for magnesium oxide and sodium oxide, and explaining the rationale behind the chemical formula of sodium oxide.
🔍 Exceptions to the Octet Rule and Expanding the Octet
This section discusses exceptions to the octet rule, where molecules like beryllium dichloride and boron trifluoride have central atoms with fewer than eight electrons. It explains that these electron-deficient molecules are stabilized through different mechanisms, such as polymeric forms or dimeric structures with dative covalent bonds. The lecture also addresses the concept of octet expansion for elements in periods three and above, which can accommodate more than eight electrons due to the availability of 3d orbitals.
🌐 Octet Expansion and Electron Configurations in Compounds
The lecture explores octet expansion further, providing examples of molecules like PCl5 and SF4, where central atoms have more than eight electrons due to the availability of 3d orbitals. It contrasts this with period two elements, which cannot expand their octet due to the lack of accessible 3d orbitals. The lecture also discusses molecules with unpaired electrons, such as NO2 and ClO2, where the least electronegative element tends to have the unpaired electrons, making them radicals and highly reactive.
📘 Guidelines for Drawing Dot and Cross Diagrams
This part of the lecture offers guidelines for drawing dot and cross diagrams, particularly for polyatomic ions like sulfate, nitrate, and ammonium. It provides a set of rules for determining the central atom, distributing charges, and ensuring that terminal atoms achieve octets. The lecture also discusses exceptions to these guidelines and emphasizes the importance of practice in drawing accurate diagrams.
🤝 Coordinate Covalent Bonds and Their Representation
The lecture introduces coordinate or dative covalent bonds, where one atom donates both electrons for a single bond, often to an atom with an empty orbital. It uses ammonium and Al2Cl6 as examples, explaining how to represent these bonds in dot and cross diagrams and structural formulas. The lecture also discusses the formation of dative covalent bonds in molecules like NO2 and ClF4-, where they help achieve stable electron configurations.
🧩 Drawing Dot and Cross Diagrams for Complex Ions
This section demonstrates how to draw dot and cross diagrams for complex ions, such as the carbonate ion and the perchlorate ion. The lecture shows the process of assigning electrons to oxygen atoms to form double bonds and distributing extra electrons from external sources. It also explains how to represent the sodium ion, which donates electrons to the carbonate ion.
🔚 Conclusion of Section Five and Transition to Section Six
The lecture concludes the discussion on dot and cross diagrams and introduces the next topic, which will cover sections six and onwards. It summarizes the importance of understanding covalent bonding and the ability to draw dot and cross diagrams as a foundation for further studies in chemistry.
Mindmap
Keywords
💡Covalent Bonding
💡Sigma Bond
💡Pi Bond
💡Orbital Overlap
💡Atomic Structure
💡Electronegativity
💡Bond Energy
💡Homoatomic Molecules
💡Lewis Structure
💡Octet Rule
💡Dative Covalent Bond
Highlights
Covalent bonding is an in-depth topic compared to ionic and metallic bonding, requiring attention in lectures two and three.
Section 4.1 focuses on describing covalent bonding in terms of orbital overlap, specifically S and P orbitals.
Understanding atomic structure is prerequisite for grasping covalent bonding concepts.
Covalent bonds are classified into sigma and pi bonds, formed by head-on and side-on orbital overlaps respectively.
Sigma bonds are stronger due to direct orbital overlap compared to pi bonds.
Exercise 4.1 involves identifying the number of sigma and pi bonds in CO2 molecules.
Bond energy is the energy required to break a mole of covalent bonds and is related to bond strength.
Fluorine exhibits an abnormally weak bond energy due to electronic repulsion between lone pairs.
Polar covalent bonds are stronger due to electrostatic attraction, a concept further explained in molecular orbital theory.
Bond length and covalent radius are defined, with examples from iodine and hydrogen.
Section 5 introduces dot and cross diagrams, essential for representing covalent bonds.
Dot and cross diagrams differentiate between valence electrons from metals and non-metals in ionic compounds.
Exceptions to the octet rule are discussed, with molecules like BeCl2 and BF3 being electron deficient.
Exercise 5.2 guides students in drawing dot and cross diagrams for molecules like HCl and CO2.
Octet expansion is possible for elements in period three and above, allowing more than eight electrons in the outer shell.
Exercise 5.3 involves drawing dot and cross diagrams for molecules like AlCl3 and BH3, illustrating electron deficiency.
Coordinate or dative covalent bonding is introduced, where one atom donates both electrons for a bond.
Examples of coordinate bonding include NH3BF3 and Al2Cl6, highlighting Lewis acidic and basic centers.
Exercise 5.6 challenges students to draw dot and cross diagrams for complex ions and molecules like NO2 and ClO2.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: