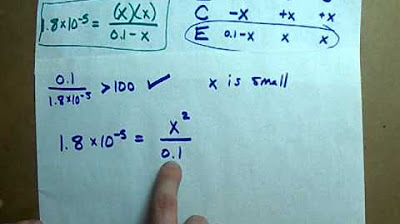Using Ka to calculate pH
TLDRThe video script explains the process of calculating pH using the acid dissociation constant (Ka), with a focus on acetic acid as an example. It outlines the dissociation equation, the initial concentration of acetic acid, and the assumption that the degree of ionization is small (less than 5%). The script details the steps to solve for the hydrogen ion concentration using the Ka expression and the assumption, then calculates the pH using the negative logarithm of the hydrogen ion concentration. The method is applicable when the ionization percentage is less than 5%, which is confirmed through a check calculation.
Takeaways
- 📚 The discussion is about calculating pH using the Ka value, specifically for acetic acid with a Ka of 1.5 or 1.8 x 10^-5.
- 🧪 The problem involves a 0.30 Molar solution of acetic acid and requires understanding its dissociation into hydrogen ions and acetate ions.
- 📊 An ICE (Initial, Change, Equilibrium) table is used to set up the reaction, with initial concentration of acetic acid being 0.3 and changes denoted by 'X'.
- 🔄 The reaction is assumed to proceed to the left, meaning the concentration of acetic acid decreases while those of hydrogen and acetate ions increase.
- 📐 The Ka expression is used to relate the concentrations: Ka = [H+][CH3COO-] / [CH3COOH].
- 🤔 The problem does not provide the pH, so the equilibrium concentrations must be determined to find the hydrogen ion concentration.
- 📈 An assumption is made that the ionization percentage is small (<5%), simplifying the calculation by considering [H+] ≈ initial concentration of acid.
- 🔢 The quadratic formula or equation solver would typically be used to find 'X' if the ionization percentage were significant, but it's not necessary in this case.
- 🎯 The calculated hydrogen ion concentration [H+] is 2.3 x 10^-3 Molar, leading to a pH of 2.6 for the solution.
- 🚨 The simplification method used is valid only when the ionization is less than 5%, which is confirmed by calculating an ionization percentage of 0.77%.
- 📝 The process demonstrated is a practical approach to solving acid dissociation problems when certain assumptions about ionization can be made.
Q & A
What is the topic of discussion in the transcript?
-The topic of discussion is how to use the Ka (acid dissociation constant) to calculate the pH of a solution, specifically using acetic acid as an example.
What is the Ka value for acetic acid mentioned in the transcript?
-The Ka value for acetic acid mentioned is 1.5 or 1.8 times 10 to the negative fifth.
What is the molarity of the acetic acid solution discussed in the transcript?
-The molarity of the acetic acid solution discussed is 0.30 M.
What is the dissociation equation for acetic acid?
-The dissociation equation for acetic acid is CH3COOH (acetic acid) → H+ (hydrogen ion) + CH3COO- (acetate ion).
What is the initial concentration represented in the ICE table for the acetic acid solution?
-The initial concentration represented in the ICE table is 0.3 M for acetic acid and 0 M for both hydrogen ions and acetate ions.
How does the assumption of a small percentage of ionization simplify the calculation?
-The assumption of a small percentage of ionization (less than 5%) simplifies the calculation by allowing us to treat the change in concentration (X) as negligible, thus not requiring the use of the quadratic formula or a calculator to solve for X.
What is the calculated hydrogen ion concentration (X) in the example?
-The calculated hydrogen ion concentration (X) in the example is 2.3 times 10 to the negative third M.
How is the pH of the solution calculated?
-The pH of the solution is calculated using the formula pH = -log[H+], where [H+] is the hydrogen ion concentration. In this case, pH = -log(2.3 × 10^-3), which results in a pH of 2.6.
What is the percentage ionization for the acetic acid solution?
-The percentage ionization for the acetic acid solution is 0.77%, which is calculated by dividing the hydrogen ion concentration at equilibrium (2.3 × 10^-3) by the initial acid concentration (0.30) and multiplying by 100.
Why is it important to check the percentage ionization?
-It is important to check the percentage ionization to ensure it is less than 5%, which validates the assumption that the change in concentration (X) is negligible. If the percentage ionization were greater than 5%, the quadratic formula or a calculator would be necessary to solve for X accurately.
What is the significance of the quadratic formula in solving this problem?
-The quadratic formula is significant in solving this problem when the percentage ionization is greater than 5% because it allows for the accurate calculation of the hydrogen ion concentration (X) from the Ka expression. However, in this example, the assumption of a small percentage ionization simplifies the process, making the quadratic formula unnecessary.
Outlines
📚 Calculating pH using KA: Introduction and Assumptions
This paragraph introduces the concept of calculating pH using the acid dissociation constant (KA) with a focus on acetic acid. It outlines the initial steps of the process, including the decomposition equation of acetic acid into hydrogen ions and acetate ions. The speaker emphasizes the typical approach of converting pH to hydrogen ion concentration but notes that this information is not provided in the current example. Instead, the speaker uses the given KA value and the initial concentration of acetic acid to set up an ICE (Initial, Change, Equilibrium) table. The key assumption made is that the ionization percentage is small enough to neglect the change in the initial concentration of the acid, simplifying the calculation process.
🧪 Solving the Problem with the Quadratic Formula and the 5% Rule
In this paragraph, the speaker discusses the algebraic approach to solving the problem without the pH value. The speaker explains how to use the KA expression and the ICE table to set up an equation, which typically requires solving using the quadratic formula or an equation solver. However, an assumption is made that the degree of ionization is less than 5%, which simplifies the calculation. The speaker demonstrates how to apply this assumption to approximate the hydrogen ion concentration and subsequently calculate the pH using the negative logarithm. The speaker also emphasizes the importance of verifying that the assumption holds by calculating the percentage of ionization and ensuring it is less than 5%. The explanation concludes with the solution's pH value and a reminder that this method is applicable when the ionization is below 5%, and otherwise, more complex mathematical methods would be necessary.
Mindmap
Keywords
💡Ka
💡pH
💡Acetic Acid
💡Dissociation
💡Equilibrium
💡Hydrogen Ion Concentration
💡Quadratic Formula
💡Percentage Ionization
💡Logarithm
💡Conjugate Base
💡ICE Table
Highlights
Introduction to calculating pH using Ka
Example problem with acetic acid and its Ka value
Decomposition equation for acetic acid
Initial concentration and ICE table setup
Assumption for small percentage of ionization
Simplification of the Ka expression
Algebraic approach without quadratic formula
Calculation of hydrogen ion concentration
Determination of pH using logarithm
Verification of the ionization percentage
Practical application of the 5% rule
Conclusion on solving the problem
Explanation of the 5% rule's applicability
Alternative methods for solving with higher ionization percentages
Relevance of the quadratic formula and graphing calculator in exams
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: