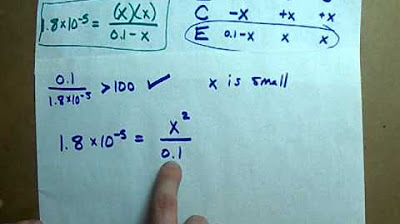Acid Dissociation Constant, Ka and pKa (A-Level Chemistry)
TLDRThis video delves into the concept of the acid dissociation constant (Ka), explaining its role in determining the strength of weak acids in solution. It contrasts weak and strong acids, highlighting the partial dissociation of the former and the complete dissociation of the latter. The video also introduces the dynamic equilibrium concept, the calculation of Ka, and its relationship with pH. The use of the PKA value for easier calculations is mentioned, along with the method to determine the concentration of H+ ions using Ka, and the assumption that the initial concentration of a weak acid is equivalent to its concentration at equilibrium.
Takeaways
- 📚 The concept of acid dissociation constant (Ka) is introduced as a measure to quantify the strength of a weak acid.
- 🔍 Understanding the Bronsted-Lowry definition of acids and bases is fundamental, where acids donate protons (H+) and bases accept them.
- 💧 When weak acids are added to water, they partially dissociate into H+ ions and their conjugate bases, establishing a dynamic equilibrium.
- ⚖️ The equilibrium constant (Ka) for a weak acid is calculated using the concentrations of the products (H+ and conjugate base) and the reactant (weak acid).
- 🔄 The position of equilibrium indicates the relative concentrations of reactants and products, with a rightward position indicating more products and a leftward position indicating more reactants.
- 🏆 The strength of an acid is directly related to the value of its Ka; a larger Ka signifies a stronger acid due to greater dissociation.
- 🌡️ Ka values are temperature-dependent, with the same acid having different Ka values at different temperatures.
- 📈 The use of the PKa value, which is the negative logarithm (base 10) of Ka, can simplify calculations and provide an alternative measure of acid strength.
- 🧪 The pH of a weak acid solution can be determined using the Ka value and the initial concentration of the weak acid, assuming minimal dissociation.
- 🔄 It's important to note that the initial concentration of weak acid is used in Ka calculations, but in practice, the equilibrium concentration is slightly different due to dissociation.
- 🎓 The video encourages further learning through related content and resources, emphasizing the importance of a comprehensive understanding of acid-base chemistry.
Q & A
What is an acid dissociation constant (Ka)?
-The acid dissociation constant (Ka) is an equilibrium constant that describes the position of equilibrium for the dissociation of a weak acid at a particular temperature, based on the concentration of weak acid (HA), hydrogen ions (H+), and conjugate base ions (A-) at equilibrium.
How do strong acids differ from weak acids in terms of dissociation in water?
-Strong acids fully dissociate when added to water, meaning virtually all the acid molecules split into H+ ions and their conjugate bases. In contrast, weak acids only partially dissociate in water, resulting in a mixture of acid molecules, H+ ions, and conjugate base ions.
What is a dynamic equilibrium and how does it relate to acid dissociation?
-A dynamic equilibrium refers to a reversible reaction in a closed system where the rates of the forward and reverse reactions are the same. In the context of acid dissociation, it means that as HA molecules dissociate into H+ and A-, the H+ and A- can also recombine to form HA, establishing an equilibrium between the reactants and products.
How is the position of equilibrium numerically represented in an equilibrium system?
-The position of equilibrium is numerically represented using equilibrium constants, such as Ka for acids. A larger Ka value indicates a stronger acid and a position of equilibrium that lies more to the right, meaning more products compared to reactants.
What is the relationship between Ka and temperature?
-The Ka value of an acid is only relevant for a specific temperature. As temperatures change, the Ka value can also change, indicating that the strength of an acid and the position of equilibrium can be temperature-dependent.
What is the pH scale and how is it related to Ka and PKa values?
-The pH scale is a logarithmic scale that measures the acidity or basicity of a solution. It is related to Ka and PKa values as the pH can be determined using the Ka value of a weak acid. PKa is the negative logarithm of Ka (base 10), and it provides an easier way to work with Ka values, especially for weak acids.
How can you calculate the pH of a weak acid solution using its Ka value?
-You can calculate the pH of a weak acid solution by using the Ka value in the expression relating the concentrations of H+, A-, and HA. By rearranging the expression and taking the square root, you can find the H+ concentration, which can then be used to calculate the pH as outlined in the formula pH = -log[H+].
What is the significance of the initial concentration of a weak acid in Ka calculations?
-In Ka calculations, the initial concentration of a weak acid is often assumed to be the same as the concentration at equilibrium because the degree of dissociation is usually very small. This simplification is made for ease of calculation, but it is important to understand that this is an assumption.
How do you determine the strength of an acid based on its Ka value?
-The strength of an acid can be determined by its Ka value. A larger Ka value indicates a stronger acid because it means more of the acid has dissociated in solution. Conversely, a smaller Ka value indicates a weaker acid with less dissociation occurring.
What is the relationship between the concentrations of H+ ions and A- ions at equilibrium for a weak acid?
-At equilibrium, the concentration of H+ ions produced from the dissociation of a weak acid (HA) is equal to the concentration of the conjugate base ions (A-). This is because for every mole of HA that dissociates, one mole of H+ and one mole of A- are released.
How can you use the Ka value to find the concentration of H+ ions in a weak acid solution?
-You can use the Ka value to find the concentration of H+ ions in a weak acid solution by rearranging the Ka expression to solve for [H+]. This involves using the initial concentration of the weak acid (HA) and the Ka value to calculate [H+], which can then be used to determine the pH of the solution.
Outlines
📚 Introduction to Acid Dissociation Constant (Ka)
This paragraph introduces the concept of the acid dissociation constant (Ka) and its significance in understanding the behavior of weak acids in solution. It explains that Ka is a measure of the extent to which a weak acid dissociates in water, forming H+ ions and its conjugate base. The paragraph also touches on the Bronsted-Lowry theory of acids and bases, emphasizing that acids are proton donors and bases are proton acceptors. It sets the stage for a deeper understanding of how the strength of an acid can be quantified using Ka values and how these values can be used to calculate the concentration of H+ ions in a solution of a weak acid. The dynamic equilibrium between reactants and products in such a system is also introduced, highlighting the reversible nature of the dissociation process and the importance of equilibrium constants in describing this relationship.
📈 Comparison of Acid Strengths and the Role of Ka
This paragraph delves into the comparison of different acids based on their Ka values. It explains that a higher Ka value indicates a stronger acid, as it dissociates more in solution, resulting in a greater concentration of H+ ions and conjugate base ions. Conversely, a lower Ka value signifies a weaker acid, with more undissociated acid molecules in the solution. The paragraph uses the example of ethanoic acid and benzoic acid to illustrate this concept, showing that ethanoic acid, with a higher Ka value, is a stronger acid than benzoic acid. It also contrasts weak acids with strong acids, which have very high Ka values and effectively dissociate completely in water. The paragraph further introduces the concept of pKa, which is the negative logarithm of Ka, providing a more manageable value for calculations and comparisons.
🧪 Practical Application: Calculating H+ Concentration and pH
The final paragraph focuses on the practical application of Ka values in determining the H+ concentration and pH of a weak acid solution. It outlines the method for calculating the H+ concentration using the Ka expression, which involves the initial concentration of the weak acid. The paragraph clarifies that, despite the slight decrease in the concentration of the weak acid due to dissociation, it is commonly assumed that the initial concentration is equal to the equilibrium concentration for simplification in calculations. This assumption is valid because the degree of dissociation is typically small. The paragraph concludes by emphasizing the utility of Ka values in understanding the behavior of weak acids in solution and provides guidance on further resources for learning about related topics such as pH calculation.
Mindmap
Keywords
💡Acid dissociation constant (Ka)
💡Weak acid
💡Conjugate base
💡Dynamic equilibrium
💡Equilibrium constant
💡pH scale
💡Bronted-Lowry theory
💡Strong acid
💡pKa
💡Hydrogen ion concentration
💡Initial concentration
Highlights
The introduction of the acid dissociation constant (Ka) and its significance in understanding the behavior of weak acids in solution.
Explanation of how to calculate acid association constants and use their values to find the concentration of H+ ions in a weak acid solution.
The distinction between strong and weak acids in terms of their degree of dissociation in water.
Dynamic equilibrium and its relevance to reversible reactions in a closed system, maintaining constant concentrations of reactants and products.
The concept of the position of equilibrium and how it relates to the concentrations of reactants and products in a mixture.
The use of equilibrium constants to numerically represent the relationship between reactants and products at equilibrium.
The formula for calculating the equilibrium constant of a weak acid and its components.
The impact of temperature on the Ka value of an acid, highlighting that Ka values are specific to a particular temperature.
Comparison of Ka values for different acids, such as ethanoic acid and benzoic acid, to illustrate their relative strengths.
The significance of the Ka value in determining the concentration of H+ ions and the conjugate base in a solution.
Introduction of the PKA value as a convenient alternative to Ka, using the logarithm to base 10 for easier calculations.
The method for calculating the pH of a weak acid solution using the Ka value and the initial concentration of the weak acid.
The assumption that the initial concentration of a weak acid is equivalent to its concentration at equilibrium for the purpose of Ka calculations.
The conclusion that summarizes the formation of an equilibrium system when weak acids dissolve in water, the role of Ka in describing this equilibrium, and the method for finding H+ concentration.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: