Calculate Ka from pH ICE Table
TLDRThe video script discusses the process of determining the ionization constant (Ka) for a weak acid, butanoic acid, using an ICE table and pH value. The explanation involves the initial molar concentration of butanoic acid, the equilibrium established upon reaction with water, and the final pH measurement to calculate the hydronium ion concentration. By applying the ICE table method and substituting values into the equilibrium expression, the Ka value is derived, indicating the acid's strength. The video encourages further exploration of acid-base equilibrium through additional examples and resources.
Takeaways
- 🧪 The problem involves butanoic acid reacting with water to form a butane and hydronium ion, which is a weak acid reaction.
- 📉 Given data includes an initial molar concentration of butanoic acid at 8.05 M and a pH of 2.72 at equilibrium.
- 🔍 The goal of the problem is to find the ionization constant (Ka) for the acid reaction.
- 📚 ICE table (Initial, Change, Equilibrium) is a method used to keep track of concentrations during the reaction.
- 👉 Three crucial pieces of information are required for ICE table: equilibrium constant (Ka), initial concentration, and final concentrations at equilibrium.
- 🦁 Butanoic acid is a weak acid and only partially ionizes in water, with an estimated 5% ionization.
- 📈 pH is used to determine the concentration of hydronium ions at equilibrium, calculated as 10^(-pH).
- 🧠 The ICE table helps in finding the change in concentrations of reactants and products during the reaction.
- 🔄 At equilibrium, the rate of reactants converting to products equals the rate of products converting back to reactants, resulting in constant concentrations.
- 🧮 The Ka value is calculated as the ratio of the product of the concentrations of the products to the concentration of the reactants at equilibrium.
- 🎓 The calculated Ka for butanoic acid in this problem is 6.87 × 10^(-5), indicating a relatively strong acid for a weak acid.
Q & A
What is the main topic of the script?
-The main topic of the script is the process of performing an ICE (Initial, Change, Equilibrium) table for an acid-base reaction involving butanoic acid and water, and solving for the ionization constant (Ka).
What is pH and how is it related to the concentration of hydrogen ions?
-pH is a measure of the hydrogen ion concentration in a solution. It is defined as the negative logarithm (base 10) of the hydrogen ion concentration. A lower pH value indicates a higher concentration of hydrogen ions, making the solution more acidic.
What is the initial molar concentration of butanoic acid given in the problem?
-The initial molar concentration of butanoic acid given in the problem is 8.05 M (moles per liter).
What is the pH at equilibrium for the given reaction?
-The pH at equilibrium for the given reaction is 2.72.
How can you use the pH value to find the concentration of hydronium ions at equilibrium?
-You can use the pH value to find the concentration of hydronium ions at equilibrium by using the formula: hydrogen ion concentration = 10^(-negative pH value). For example, at a pH of 2.72, the hydrogen ion concentration is 10^(-2.72) M.
What is the role of the ICE table in solving acid-base equilibrium problems?
-null
What is the ICE table in the context of the script?
-The ICE table is a method used in chemistry to keep track of the initial concentrations, changes in concentrations, and equilibrium concentrations of reactants and products in a reaction. It helps in setting up and solving for the equilibrium constant (Ka) of an acid-base reaction.
What does the equilibrium constant (Ka) represent in the context of the acid-base reaction?
-The equilibrium constant (Ka) represents the ratio of the concentration of products to the concentration of reactants at equilibrium for an acid-base reaction. It is a measure of the extent to which the reaction favors the formation of products over reactants at equilibrium.
How does the ICE table help in finding the unknown Ka value?
-The ICE table helps in finding the unknown Ka value by providing a systematic way to calculate the changes in concentrations of reactants and products as the reaction moves towards equilibrium. By setting up the table with the initial and equilibrium concentrations, and knowing the reaction stoichiometry, one can solve for the Ka value.
What is the significance of the ICE table in solving for the Ka value?
-The ICE table is significant in solving for the Ka value as it organizes the information about the reaction and allows for a clear visualization of the changes in concentrations from the start to the equilibrium. This organization is crucial for accurately setting up and solving the equation for the Ka value.
What is the relationship between the pH value and the Ka value?
-The pH value is directly related to the Ka value in that the pH at equilibrium can be used to calculate the Ka value for a weak acid. The lower the pH, the higher the concentration of hydrogen ions, which indicates a higher Ka value, meaning the acid is more ionized and thus stronger.
How does the ICE table help in understanding the extent of ionization of a weak acid?
-The ICE table helps in understanding the extent of ionization of a weak acid by showing the initial and equilibrium concentrations of the acid and its conjugate base. By comparing these concentrations, one can see the extent to which the weak acid has ionized at equilibrium.
What is the role of the ICE table in determining the equilibrium concentrations of reactants and products?
-The ICE table plays a crucial role in determining the equilibrium concentrations of reactants and products by allowing us to visualize and calculate the changes in concentration as the reaction moves towards equilibrium. This helps in setting up the equation to solve for the equilibrium constant (Ka) and understand the reaction's behavior at equilibrium.
Outlines
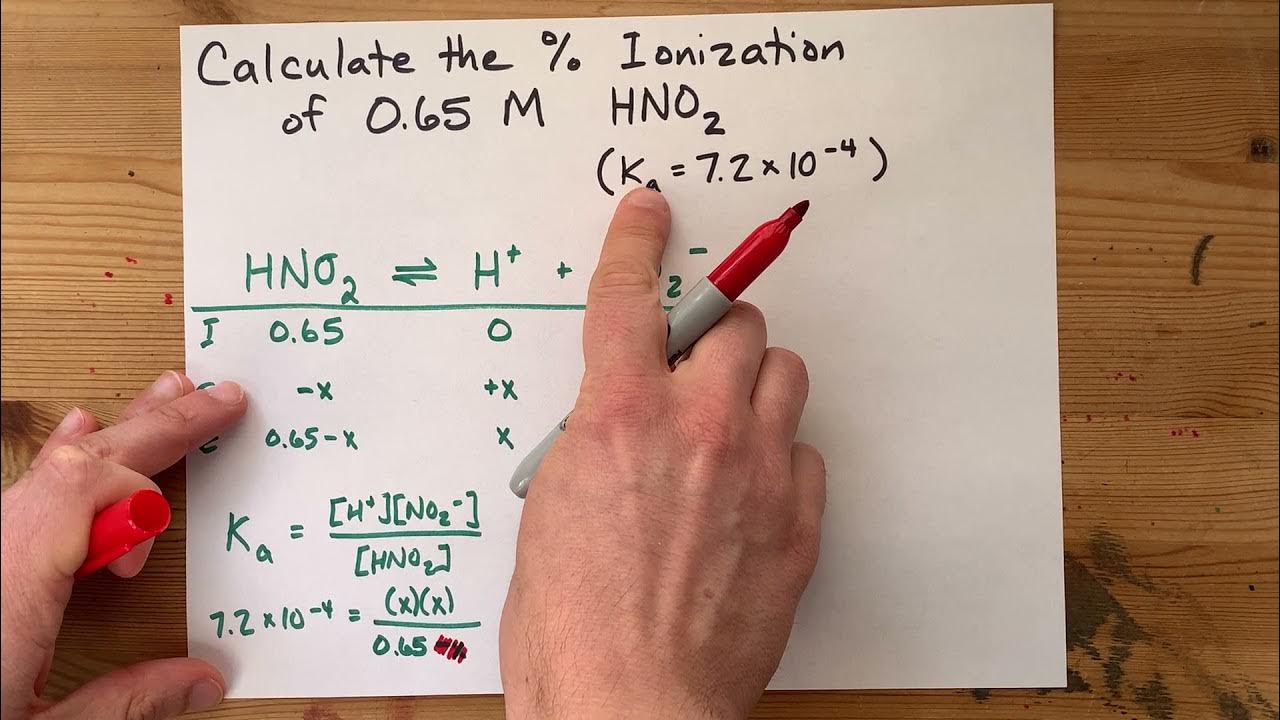
🧪 Introduction to Acid-Base Reactions and ICE Table
This paragraph introduces the concept of acid-base reactions, specifically focusing on butanoic acid reacting with water to form a conjugate base and hydronium ion. The importance of pH in determining the equilibrium constant (Ka) for the reaction is emphasized. The speaker explains the problem setup, which involves an initial molar concentration of butanoic acid and a final pH of 2.72. The ICE table method is introduced as a tool to solve for the unknown Ka, with the three crucial pieces of information being the equilibrium constant, initial concentration, and final concentrations at equilibrium.
📊 Calculation of Ka using pH and ICE Table
The speaker continues by detailing the process of using the ICE table to calculate the ionization constant (Ka) for the acid reaction. They explain how to use the given pH to find the hydronium ion concentration at equilibrium and how this relates to the equilibrium concentrations of the reactants and products. The ICE table is filled out with the initial and change in concentrations, leading to the calculation of the final concentrations. The speaker then plugs these values into the equilibrium expression to solve for Ka, resulting in a Ka value of 6.87 x 10^-5. The significance of this value in the context of the strength of the acid is discussed, and the speaker encourages further study of acid-base equilibria through additional examples and playlists.
Mindmap
Keywords
💡Acid-Base Reaction
💡pH
💡Equilibrium Constant (Ka)
💡ICE Table
💡Conjugate Base
💡Hydronium Ion
💡Molarity
💡Partial Ionization
💡Ionization Constant
💡Weak Acid
Highlights
Excitement about conducting an ice table with an acid reaction experiment.
The importance of pH in determining acid-base reactions and equilibrium constants.
Butanoic acid reacting with water to form butane as the conjugate base.
Initial molar concentration of butanoic acid given as 8.05 molar.
Equilibrium achieved with a pH probe showing a pH of 2.72.
The objective to find the ionization constant (Ka) for the acid reaction.
Explanation of the three crucial pieces of information needed for ICE tables: equilibrium constant, initial concentration, and final concentrations at equilibrium.
Weak acids like butanoic acid only partially ionize, with a guessed 5% reaction rate.
The establishment of beautiful equilibrium where the rates of reactants to products and vice versa become constant.
Using pH to find the concentration of hydronium ions at equilibrium, with the formula hydrogen concentration equal to 10 to the negative pH.
The ICE table setup with water as a liquid and butanoic acid, butane, and hydronium ion concentrations.
The calculation of changes in concentrations based on stoichiometric coefficients and the establishment of final concentrations at equilibrium.
The derivation of Ka from the equilibrium concentrations and the calculation of Ka for butanoic acid.
Ka represents the ratio of product concentrations to reactant concentrations at equilibrium, signifying the strength of the acid.
The significance of the calculated Ka value, 6.87 times 10 to the minus 5, in characterizing the acid strength of butanoic acid.
Encouragement for further practice and study of acid-base equilibria through additional examples and resources.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: