Chapter 6: Reaction Quotient | CHM 214 | 052
TLDRThe transcript discusses the concept of the reaction quotient (q) and its role in understanding non-equilibrium systems. It explains that q, similar to the equilibrium constant (k), is calculated using concentrations of reactants and products. However, unlike k, which is a fixed constant at equilibrium, q can vary and is initially zero when no products are formed. The script emphasizes that reactions adjust until q equals k, with the direction of the reaction depending on whether q is less than or greater than k, aligning with the Le Chatelier principle.
Takeaways
- 📈 The reaction quotient (Q) is similar to the equilibrium constant (K) but is used for systems not at equilibrium.
- 🔄 Q can have any value for non-equilibrium systems, whereas K is a fixed constant for a given reaction at equilibrium.
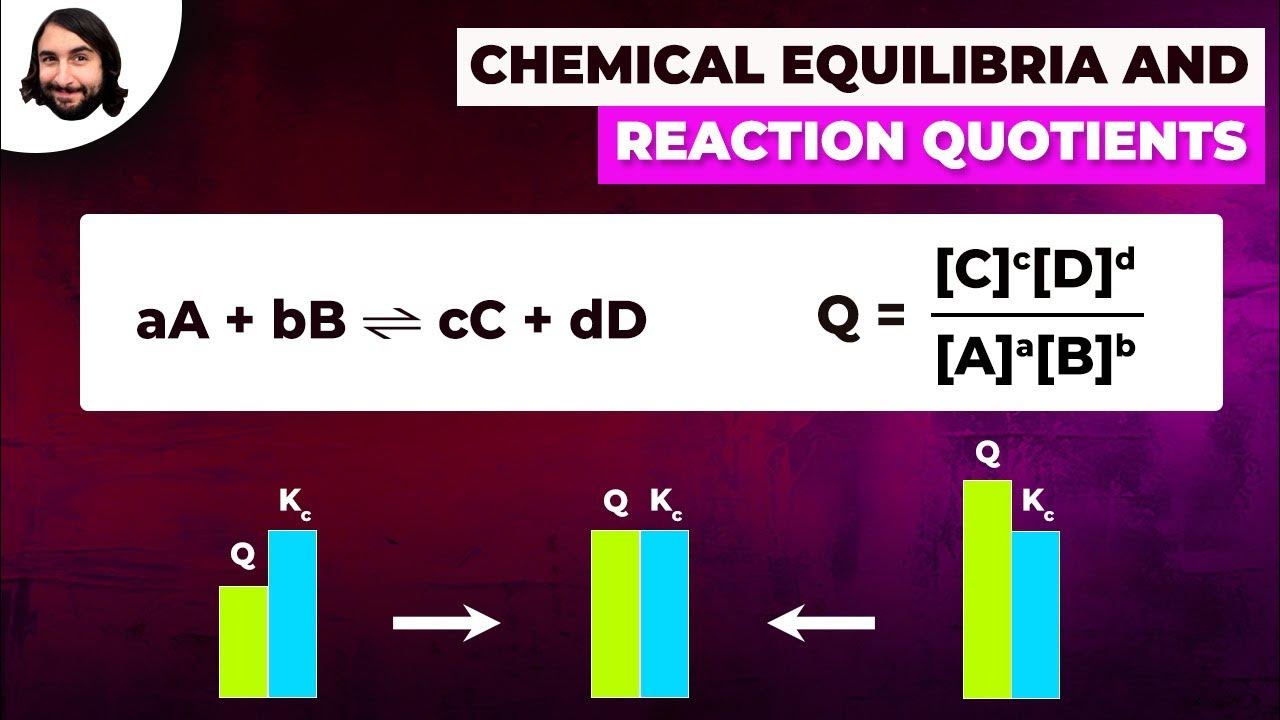
- 🌐 At the start of a reaction with no products formed, Q is equal to 0, unlike K which can never be zero.
- 🔄 The value of Q changes as the reaction progresses, aiming to reach the value of K to establish equilibrium.
- 🠗 If Q is less than K, the reaction will proceed to the right, increasing the concentration of products (C and D).
- 🠕 If Q is greater than K, the reaction will proceed to the left, decreasing the concentration of products or increasing the concentration of reactants (A and B).
- ⚖️ The relationship between Q and K is crucial in determining the direction in which a reaction will proceed.
- 🧪 The concept of Q and K applies to the Le Chatelier's principle, which states that a system will adjust to counteract changes in conditions.
- 🏋️♂️ Regardless of the size of K, the reaction will always strive to reach equilibrium by adjusting concentrations.
- 🔄 The adjustment of Q towards K is a dynamic process that continues until equilibrium is reached.
- 📚 Understanding the interplay between Q, K, and reaction progress is fundamental in the study of chemical equilibria.
Q & A
What is the main difference between the equilibrium constant (K) and the reaction quotient (Q)?
-The equilibrium constant (K) applies to systems that are already at equilibrium, representing a fixed value that indicates the ratio of product concentrations to reactant concentrations at equilibrium. In contrast, the reaction quotient (Q) is used for systems that are not at equilibrium and can vary as the reaction proceeds, reflecting the current ratio of product to reactant concentrations at any given time.
How does the reaction quotient (Q) change as a reaction moves towards equilibrium?
-As a reaction proceeds, the value of Q will change. If Q is less than K, the reaction will proceed in the forward direction to produce more products (numerator increases) until Q equals K. If Q is greater than K, the reaction will proceed in the reverse direction to consume excess products and produce more reactants (numerator decreases or denominator increases) until Q equals K.
What is the significance of the reaction quotient (Q) being equal to zero in a system?
-A reaction quotient (Q) of zero indicates that no products have been formed yet in the reaction. This situation occurs when reactants are mixed but no reaction has occurred, and it signifies the initial stage of a reaction where only reactants are present.
Can the value of the equilibrium constant (K) ever be zero?
-The equilibrium constant (K) can never be exactly zero. It can be very small, indicating that the reaction strongly favors the reactants, but it will not be zero because it represents a ratio of concentrations, and there is always some finite probability that products will be present at equilibrium.
What is Le Chatelier's Principle, and how does it relate to the relationship between Q and K?
-Le Chatelier's Principle states that if a system at equilibrium is subjected to a change in conditions, the system will adjust to counteract the change and re-establish equilibrium. This principle applies to the relationship between Q and K, as reactions will proceed in a direction that will change the value of Q to match the value of K, the equilibrium constant, thereby maintaining or re-establishing equilibrium.
How does the value of Q influence the direction in which a reaction will proceed?
-The value of Q determines the direction in which a reaction will proceed to reach equilibrium. If Q is less than K, the reaction will proceed in the forward direction to increase the concentration of products. If Q is greater than K, the reaction will proceed in the reverse direction to increase the concentration of reactants.
What happens to the reaction quotient (Q) as a reaction approaches equilibrium?
-As a reaction approaches equilibrium, the value of the reaction quotient (Q) will gradually change until it equals the equilibrium constant (K). At equilibrium, the concentrations of reactants and products no longer change, and Q remains constant at the value of K.
How does the equilibrium constant (K) guide a reaction towards equilibrium?
-The equilibrium constant (K) is a measure of the extent to which a reaction favors the formation of products over reactants. A reaction will proceed in the direction that reduces the discrepancy between the current reaction quotient (Q) and the equilibrium constant (K), ultimately reaching a state where Q equals K at equilibrium.
What is the role of a catalyst in the equilibrium system?
-A catalyst speeds up the rate of both the forward and reverse reactions in an equilibrium system by lowering the activation energy required for each direction. However, it does not change the equilibrium constant (K), as it does not affect the relative concentrations of reactants and products at equilibrium.
How does the reaction quotient (Q) relate to the concentrations of reactants and products in a chemical reaction?
-The reaction quotient (Q) is calculated by taking the product of the concentrations of the products raised to the power of their stoichiometric coefficients and dividing by the product of the concentrations of the reactants raised to their stoichiometric coefficients. Q reflects the relative amounts of reactants and products at a specific point in time and is used to predict the direction in which a reaction will proceed to reach equilibrium.
What is the significance of comparing Q to K in predicting the direction of a reaction?
-Comparing the reaction quotient (Q) to the equilibrium constant (K) allows us to predict whether a reaction will proceed in the forward or reverse direction to reach equilibrium. If Q is less than K, the reaction will proceed to produce more products (forward direction). If Q is greater than K, the reaction will proceed to produce more reactants (reverse direction). This comparison is crucial for understanding the dynamics of chemical reactions and their progression towards equilibrium.
Outlines
📈 Introduction to Reaction Quotient and Equilibrium
This paragraph introduces the concept of the reaction quotient (Q) as a measure for systems that are not at equilibrium. It contrasts Q with the equilibrium constant (k), which is applicable only to systems that have reached equilibrium. The paragraph explains that Q is similar in form to k, with concentrations of reactants and products represented, but unlike k, Q can be zero at the beginning of a reaction when no products have been formed. The equilibrium constant k is described as a fixed value that the reaction seeks to achieve by adjusting the concentrations of reactants and products over time.
Mindmap
Keywords
💡equilibrium
💡reaction quotient (Q)
💡concentration
💡stoichiometric coefficients
💡chemical reaction
💡equilibrium constant (K)
💡dynamic condition
💡Le Chatelier's Principle
💡forward reaction
💡reverse reaction
💡stoichiometry
Highlights
k is a useful quantity for systems at equilibrium.
Reaction quotient (q) is defined for systems not at equilibrium.
The reaction quotient q is similar to the equilibrium constant k.
The concentration terms in q are the same as those in k, but q is not at equilibrium.
At the start of a reaction with no products formed, q will be equal to 0.
The value of k for any reaction can never be completely zero.
k is a constant, hence the term equilibrium constant.
A reaction will adjust until the value of q equals k.
If q is less than k, the reaction proceeds to the right to increase the concentration of products.
If q is greater than k, the reaction proceeds to the left, favoring the reactants.
The relationship between q and k holds true regardless of the size of k.
Reactions proceed to match the concentrations to the value of the equilibrium constant.
This concept applies to the well-known principle of the Chat, Liaise principle in chemistry.
The Chat, Liaise principle states that q will adjust until it equals k.
The direction of the reaction is determined by the relative values of q and k.
The concept of q and k is fundamental in understanding reaction dynamics.
The equilibrium constant k is a measure of the spontaneity of a reaction at equilibrium.
The reaction quotient q provides insight into the progress and direction of a reaction.
Understanding q and k is crucial for predicting the outcome of chemical reactions.
Transcripts
Browse More Related Video

Introduction to reaction quotient Qc | Chemical equilibrium | Chemistry | Khan Academy

Worked example: Using the reaction quotient to predict a pressure change | Khan Academy

Equilibrium

ALEKS: Using an equilibrium constant to predict the direction of a spontaneous reaction

18. Introduction to Chemical Equilibrium
Chemical Equilibria and Reaction Quotients
5.0 / 5 (0 votes)
Thanks for rating: