Equilibrium
TLDRIn this chemistry essentials video, Mr. Andersen explores the concept of chemical equilibrium through a water transfer demonstration, illustrating how reactants and products reach a balanced state where their rates of conversion into each other are equal. He explains the equilibrium constant (K) and its calculation, using the Haber Process for synthesizing ammonia as an example. The video also covers how to predict the direction of a reaction before equilibrium is reached by comparing the reaction quotient (Q) to K. Mr. Andersen demonstrates how to use stoichiometry in an ICE table (Initial, Change, Equilibrium) to solve for equilibrium concentrations and emphasizes the importance of understanding the relationship between Q and K to determine the reaction's direction. The video concludes by encouraging viewers to apply these concepts to interpret graphs and predict reaction outcomes.
Takeaways
- 🧪 Chemical equilibrium is a state where the rate of the forward reaction equals the rate of the reverse reaction, resulting in a constant amount of reactants and products.
- 📊 The equilibrium constant (K) is the ratio of the concentration of products to the concentration of reactants, raised to the power of their stoichiometric coefficients.
- ⚖️ At equilibrium, the value of K remains constant, regardless of the initial concentrations of reactants and products.
- ⏱️ The concept is demonstrated through a water transfer experiment, where water moves back and forth between two jars, eventually reaching a point where the amount moved in both directions is equal.
- 📈 The concentration of reactants and products over time in a reversible reaction will eventually level off, indicating equilibrium has been reached.
- 🔁 The Haber Process, which produces ammonia from nitrogen and hydrogen, is used as an example to illustrate the concept of chemical equilibrium.
- 📉 The rate of the forward and reverse reactions can be graphed over time, showing that at equilibrium these rates converge, indicating no net change in the concentrations of reactants and products.
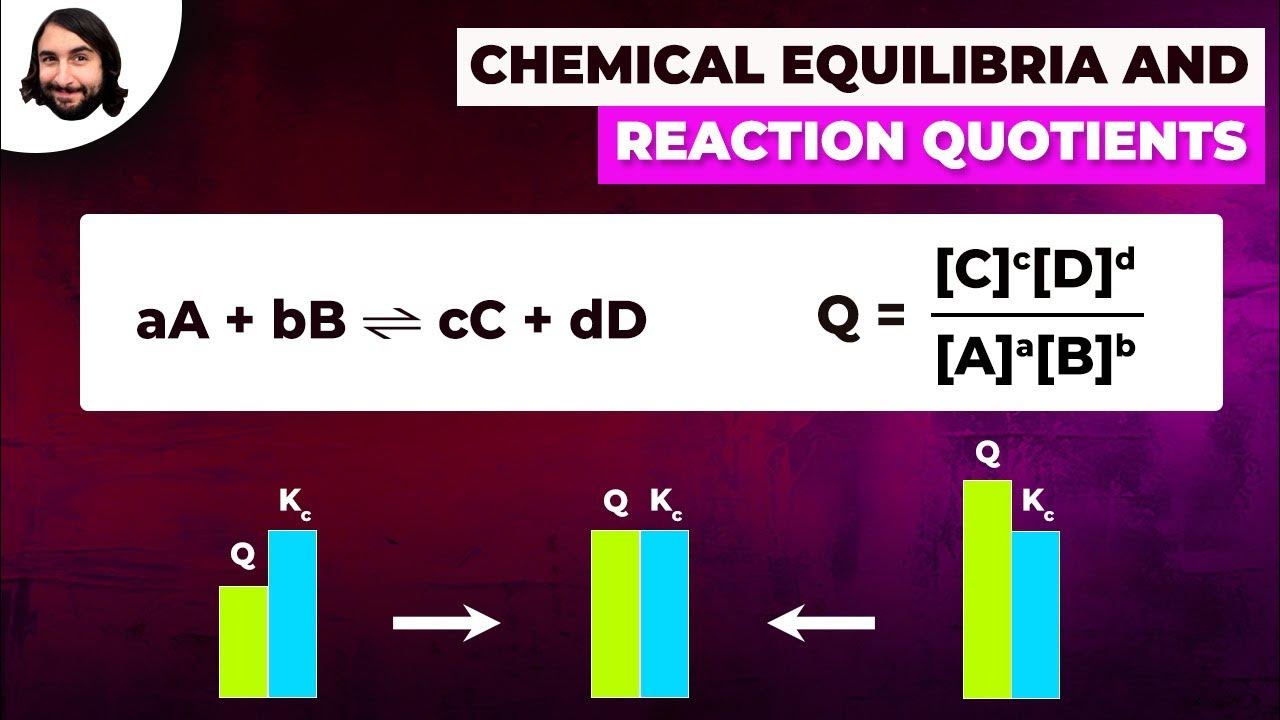
- 🔢 The reaction quotient (Q) is calculated in a similar way to K but using the initial concentrations of reactants and products. If Q is less than K, the reaction will proceed to the right (towards products).
- 🔄 An ICE table (Initial, Change, Equilibrium) is a useful tool for predicting the direction a reaction will take and for calculating the concentrations at equilibrium.
- 🔢 If Q is greater than K, the reaction will proceed to the left (towards reactants), and if Q equals K, the system is already at equilibrium.
- 🧮 The stoichiometry of the reaction determines the changes in concentrations of reactants and products in the ICE table, with the molar ratios guiding these changes.
Q & A
What is the main topic of the video?
-The main topic of the video is chemical equilibrium, which is demonstrated through an experiment involving the transfer of water between two containers to represent reactants and products.
What does the demonstration with water in two jars represent?
-The demonstration represents the concept of chemical equilibrium where reactants are converted into products and vice versa until an equilibrium state is reached, where the rates of the forward and reverse reactions are equal.
What is the equilibrium constant (K) in the context of the video?
-The equilibrium constant (K) is a measure of the ratio of the concentration of products to the concentration of reactants at equilibrium, which remains constant as long as the system is at equilibrium.
How is the equilibrium constant calculated?
-The equilibrium constant is calculated by taking the ratio of the concentrations of products to the concentrations of reactants, each raised to the power of their stoichiometric coefficients in the balanced chemical equation.
What is the significance of comparing Q and K values?
-Comparing Q (the reaction quotient calculated before equilibrium is reached) and K values allows us to predict the direction in which the reaction will proceed to reach equilibrium. If Q is less than K, the reaction will proceed to the right (toward products), and if Q is greater than K, it will proceed to the left (toward reactants).
What is the Haber Process mentioned in the video?
-The Haber Process is a method of synthesizing ammonia from nitrogen and hydrogen gases. It is an example of a reversible reaction that reaches an equilibrium state.
How does the rate of a reaction change as it reaches equilibrium?
-As a reaction reaches equilibrium, the rate of the forward reaction (reactants to products) becomes equal to the rate of the reverse reaction (products to reactants), causing the concentrations of reactants and products to remain constant over time.
What is an ICE table?
-An ICE table is a method used to calculate the concentrations of reactants and products at equilibrium. It stands for Initial, Change, and Equilibrium, and it helps to systematically organize the stoichiometry and concentrations at different stages of the reaction.
How can you determine the direction of a reaction without calculating Q and K values?
-You can determine the direction of a reaction by observing the stoichiometry of the reactants and products in the balanced chemical equation. If there are more moles of products than reactants, the reaction will favor the formation of reactants, and vice versa.
What is stoichiometry and how does it relate to chemical equilibrium?
-Stoichiometry is the quantitative relationship between reactants and products in a balanced chemical equation. It is crucial in determining the direction of a reaction and the concentrations at equilibrium, as the molar ratios dictate how much of each substance will be present at equilibrium.
How can you use the equilibrium constant to predict the extent of a reaction at equilibrium?
-The equilibrium constant (K) can be used to predict the extent of a reaction at equilibrium by providing a ratio of the concentrations of products to reactants. A large K value indicates that the reaction favors the formation of products, while a small K value indicates a preference for reactants.
Outlines
🧪 Introduction to Chemical Equilibrium
Mr. Andersen begins the video with a demonstration of chemical equilibrium using water in jars to represent reactants and products. He explains that in a reversible reaction, reactants are converted into products and vice versa, eventually reaching an equilibrium state where the rate of the forward and reverse reactions are equal. The equilibrium constant (K) is introduced as a measure of this state, calculated as the concentration of products divided by the concentration of reactants. The concept of comparing Q (the reaction quotient before equilibrium is reached) to K to predict the direction of the reaction is also discussed, with the Haber Process for ammonia production used as an example.
🔍 Calculating Equilibrium Constants and Q Values
The video continues with a deeper exploration of calculating K values, emphasizing the importance of understanding the stoichiometry of the reaction. Mr. Andersen illustrates how to calculate K using molar values at equilibrium and introduces the concept of an ICE (Initial, Change, Equilibrium) table to track these values. He demonstrates how to determine the direction a reaction will proceed based on the comparison of Q and K values and explains how to solve for the equilibrium concentrations when given initial concentrations and the equilibrium constant. The role of stoichiometry in guiding the ICE table calculations is highlighted.
📊 Analyzing Reaction Direction and Equilibrium
In the final paragraph, Mr. Andersen challenges viewers to apply their understanding of chemical equilibrium by analyzing graphs and data to predict the direction of reactions and calculate equilibrium constants. He emphasizes the ability to determine whether a system has reached equilibrium and which direction the reaction will proceed based on the concentration of reactants and products. The video concludes with a summary of key learning objectives, including interpreting graphs, predicting reaction directions, and calculating K values to understand the conditions at equilibrium.
Mindmap
Keywords
💡Chemical Equilibrium
💡Reactants and Products
💡Reversible Reaction
💡Equilibrium Constant (K)
💡Quadratic Equation
💡Stoichiometry
💡ICE Table
💡Haber Process
💡Concentration
💡Direction of Reaction
💡Q Value
Highlights
Demonstrates chemical equilibrium using water transfer between jars to represent reactants and products
Equilibrium is reached when the rate of reactants converting to products equals the rate of products converting back to reactants
Equilibrium constant (K) is the ratio of product concentration to reactant concentration at equilibrium
Can calculate K value by taking ratio of moles of products to moles of reactants at equilibrium
Q value is the same as K value before equilibrium is reached
Compare Q and K values to predict direction of reaction before equilibrium - if Q < K, reaction proceeds to the right (toward products)
Example: Haber process for synthesizing ammonia from nitrogen and hydrogen gas
Graph shows concentration of reactants and products over time, reaching a plateau at equilibrium
Different graph shows forward and reverse reaction rates converging at equilibrium
K value remains constant as long as the system is at equilibrium
Calculate Q value before equilibrium based on current concentrations of reactants and products
If Q < K, reaction proceeds to the right (toward products). If Q > K, reaction proceeds to the left (toward reactants)
Use a number line to visualize the relationship between Q, K and the direction of reaction
Write the expression for K value based on the balanced chemical equation and the moles of each species
Example calculation of K value for the Haber process using molar concentrations at equilibrium
ICE table shows initial concentrations, changes, and equilibrium concentrations
Use stoichiometry to determine the changes in concentrations of each species in the ICE table
If given initial concentrations, Q, and K, can solve for the equilibrium concentrations by setting up an equation and solving for x
Quadratic equation may be necessary if the equation does not simplify nicely, but often it can be simplified by taking square roots
Once x is found, fill in the equilibrium concentrations in the ICE table
Can read graphs to determine if a system has reached equilibrium and predict the direction of reaction based on Q and K values
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: