ALEKS: Using an equilibrium constant to predict the direction of a spontaneous reaction
TLDRThis video tutorial guides viewers on using the equilibrium constant (Kc) to predict the direction of spontaneous reactions in chemistry. It demonstrates the process by analyzing a balanced equation with given Kc and three sets of concentration data. The key is to calculate the reaction quotient (Q) for each set and compare it to Kc. If Q equals Kc, the system is in equilibrium with no change. If Q is greater than Kc, the reaction shifts left, decreasing products and increasing reactants. Conversely, if Q is less than Kc, the reaction moves right, increasing products and decreasing reactants. The video simplifies the process by showing calculations with stoichiometric coefficients of 1 and provides clear examples for each data set.
Takeaways
- 🧪 The video explains how to use an equilibrium constant (Kc) to predict the direction of a spontaneous chemical reaction.
- 📝 A balanced chemical equation and three sets of concentration data for reactants and products are provided in the problem.
- 🔍 The goal is to predict changes in concentration: increase, decrease, or no change for the substances involved.
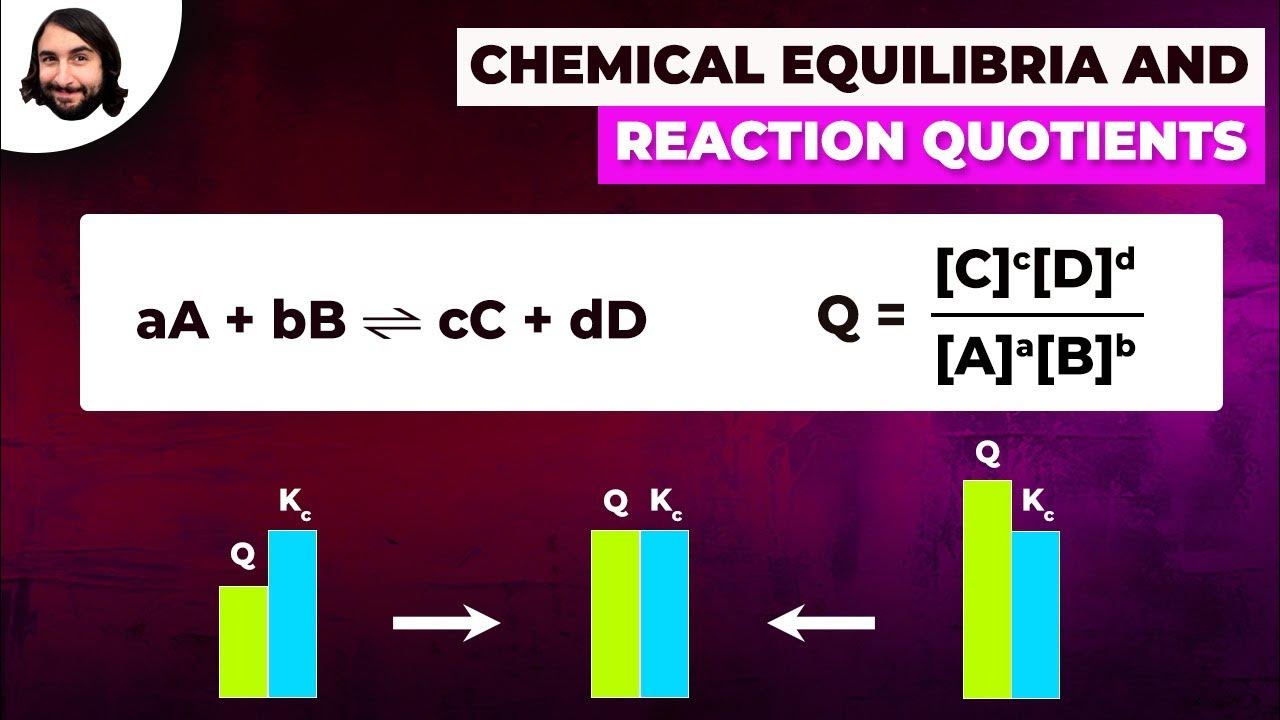
- 📚 The equilibrium expression (Q) is calculated similarly to Kc, with products over reactants, each raised to their stoichiometric coefficients.
- ⚖️ Stoichiometric coefficients are all 1 in this example, simplifying the calculation of Q.
- 🔢 The first data set involves concentrations of BF3-NH3, BF3, and NH3, which are used to calculate Q.
- 📉 For the first set, Q is calculated to be 16.8, which is compared to the given Kc value of 1.1 to determine the reaction direction.
- ➡️ If Q > Kc, the reaction is too far to the right, indicating an excess of product and a need to shift the reaction to the left.
- 🔄 A shift to the left means decreasing the product concentration and increasing the reactant concentration.
- 🔄 Conversely, if Q < Kc, the reaction would need to shift to the right, increasing product and decreasing reactant concentrations.
- 📝 The process is repeated for the other two data sets, with Q values calculated and compared to Kc to determine the reaction's direction.
- 📊 In the second and third data sets, Q values of 1.06 are calculated, which are equal to Kc, indicating that the reaction is at equilibrium with no change expected.
Q & A
What is the main topic discussed in the video?
-The video discusses solving an ALEKS problem involving using an equilibrium constant to predict the direction of a spontaneous reaction.
What information will be given in the problem?
-The problem will provide a balanced equation, a value of Kc, and three different sets of data including the concentrations of all reactants and products.
What is the purpose of calculating the value of Q?
-The value of Q is calculated to predict if the concentrations of substances will increase, decrease, or remain unchanged.
How is Q calculated in relation to K?
-Q is calculated in the same way as K, using an equilibrium expression with the products over the reactants, each raised to their stoichiometric coefficients.
What is the equilibrium expression used in the example?
-The equilibrium expression is Q = [BF3NH3] / ([BF3] * [NH3]).
What are the given concentrations for the first data set in the example?
-For the first data set, the concentrations are: [BF3NH3] = 1, [BF3] = 0.17, and [NH3] = 0.35.
How is the value of Q for the first data set calculated?
-Q is calculated as 1 / (0.17 * 0.35) = 16.8.
What does it mean if Q is greater than K?
-If Q is greater than K, it means the reaction has too much product, so the products need to decrease and the reactants need to increase to reach equilibrium.
What are the concentrations for the second data set, and what is the result?
-For the second data set, the concentrations are: [BF3NH3] = 0.83, [BF3] = 0.78, and [NH3] = 1. Q is calculated as 0.83 / (0.78 * 1) = 1.06, which rounds to 1.1, equal to K, meaning no change.
What are the concentrations for the third data set, and what is the result?
-For the third data set, the concentrations are: [BF3NH3] = 0.54, [BF3] = 0.68, and [NH3] = 0.81. Q is calculated as 0.54 / (0.68 * 0.81) = 1.06, which rounds to 1.1, equal to K, meaning no change.
Outlines
🧪 Understanding Spontaneous Reactions with Equilibrium Constants
This paragraph introduces a method to predict the direction of a spontaneous chemical reaction using an equilibrium constant. The video will demonstrate how to solve a problem involving a balanced chemical equation, given concentrations of reactants and products, and a value of the equilibrium constant (Kc). The process involves calculating the reaction quotient (Q) for different sets of data and comparing it to Kc. If Q equals Kc, the system is at equilibrium with no change expected. If Q is greater than Kc, the reaction will shift to the left, decreasing product concentration and increasing reactant concentration. Conversely, if Q is less than Kc, the reaction will shift to the right, increasing product concentration and decreasing reactant concentration. The first data set provided in the script is used to illustrate this process, with calculations leading to a Q value of 16.8, which is greater than the given Kc of 1.1, indicating that the reaction will proceed to decrease product concentration.
📉 Analyzing Additional Data Sets for Equilibrium
The second paragraph continues the discussion by analyzing two more data sets to determine if the reaction is at equilibrium, will proceed in the forward direction, or in the reverse direction. The calculations for the second data set result in a Q value of 1.06, which is equal to the Kc of 1.1, indicating that no change is expected as the system is already at equilibrium. The third data set also yields a Q value of 1.06, confirming equilibrium with no expected change in concentrations of reactants or products. The paragraph emphasizes the importance of comparing Q to Kc to predict the direction of a reaction and to understand the state of equilibrium.
Mindmap
Keywords
💡Equilibrium Constant (Kc)
💡Spontaneous Reaction
💡Balanced Equation
💡Concentration
💡Stoichiometric Coefficients
💡Equilibrium Expression
💡Reaction Quotient (Q)
💡Data Sets
💡Direction of Reaction
💡Increasing and Decreasing Concentrations
💡In Equilibrium
Highlights
The video demonstrates how to use an equilibrium constant to predict the direction of a spontaneous reaction.
A balanced chemical equation and a value of kc are provided.
Three different sets of data for reactant and product concentrations are given.
The task is to predict if substances will increase, decrease, or remain unchanged in concentration.
Calculating the value of q for each data set is crucial.
The equilibrium expression is written with products over reactants, each raised to their stoichiometric coefficients.
All stoichiometric coefficients in the example are 1, simplifying calculations.
The equilibrium expression is b of 3 nh3 over bf3, times nh3.
Concentration values for bf3nh3, bf3, and nh3 are plugged into the q equation.
For the first data set, the concentrations are bf3nh3 = 1, bf3 = 0.17, nh3 = 0.35.
The calculated q value is 16.8 for the first data set.
The value of k is 1.1, used to compare with q.
If q equals k, the reaction is in equilibrium with no change.
If q is greater than k, the reaction needs to shift to the left, decreasing products and increasing reactants.
For the second data set, the concentrations are bf3nh3 = 0.83, bf3 = 0.78, nh3 = 1.
The calculated q value is 1.06 for the second data set, equal to k, indicating no change.
For the third data set, the concentrations are bf3nh3 = 0.54, bf3 = 0.68, nh3 = 0.81.
The calculated q value is again 1.06 for the third data set, indicating equilibrium.
Transcripts
Browse More Related Video

Introduction to reaction quotient Qc | Chemical equilibrium | Chemistry | Khan Academy
Chemical Equilibria and Reaction Quotients

Calculating Moles at Equilibrium

Using RICE to calculate equilibrium concentrations

Chapter 6: Reaction Quotient | CHM 214 | 052

How To Calculate Kp From Kc - Chemical Equilibrium
5.0 / 5 (0 votes)
Thanks for rating: