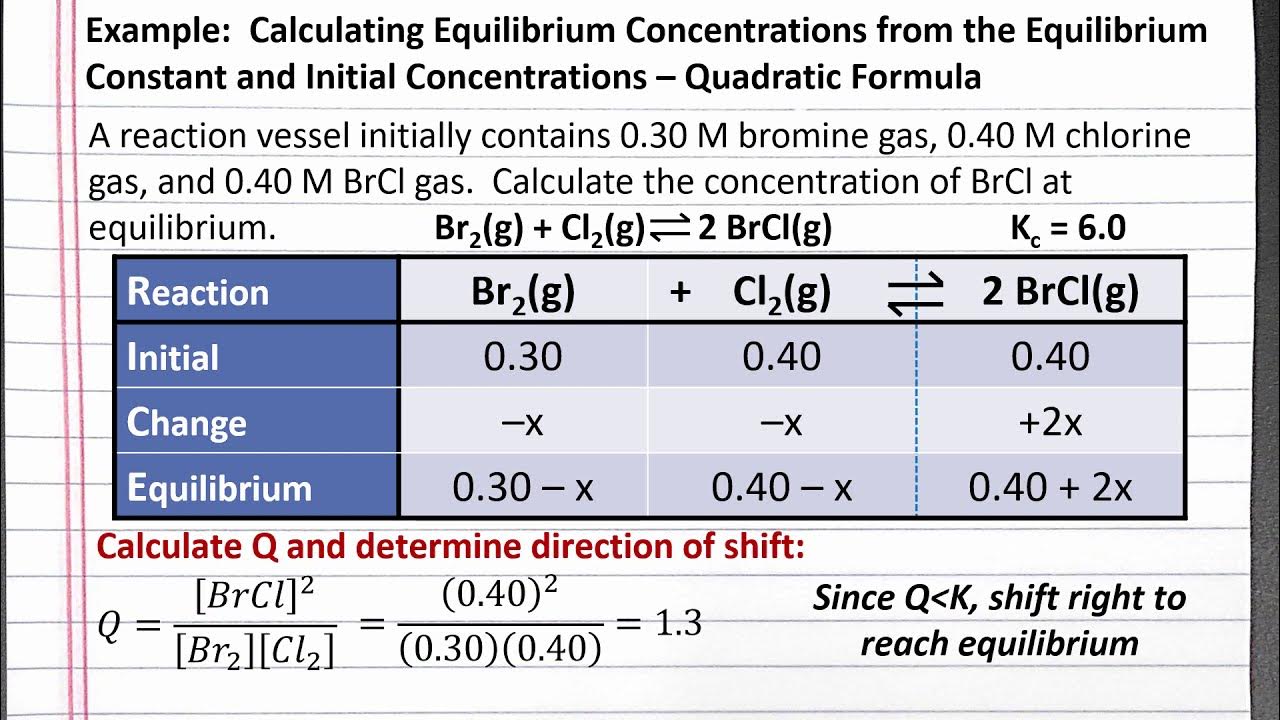
ALEKS - Calculating Equilibrium Composition from an Equilibrium Constant
TLDRThis video script outlines a chemistry lesson on calculating equilibrium composition using an equilibrium constant. The instructor walks through the steps of solving a problem involving a reaction between nitrogen and oxygen gases to form nitrogen monoxide. The unique challenge is the need to solve for 'X', which cannot be considered negligible. The script details the use of an ICE table, the equilibrium constant expression, and the application of the quadratic formula to find the equilibrium molarity of O2. The lesson emphasizes the importance of algebraic manipulation and critical thinking in chemistry.
Takeaways
- 🧪 The video is a chemistry tutorial focusing on calculating equilibrium composition from an equilibrium constant.
- 🔍 It emphasizes the importance of solving for 'x' in this problem, which is often not negligible as it is in simpler cases.
- 📚 The problem involves a reaction in a 500 mL flask with gases N2 and O2 reacting to form NO, with an equilibrium constant Kc given.
- 📈 The equilibrium constant expression is set up using molarity, considering the coefficients of the balanced chemical equation.
- 🔢 The value of Kc is 0.614, indicating a balanced equilibrium that doesn't favor one side over the other.
- 📉 The initial concentrations of N2 and O2 are converted into molarity for use in the ICE table (Initial, Change, Equilibrium).
- ⚖️ The reaction is expected to shift to the left to reach equilibrium due to the absence of O2 initially.
- 📝 An ICE table is constructed to track the changes in molarity of the reactants and products as the reaction reaches equilibrium.
- 🔑 The equilibrium expression is plugged into the Kc value to form a quadratic equation that must be solved for 'x'.
- 📱 Solving the quadratic equation can be done using a calculator with a solver function, an online math solver, or by hand using the quadratic formula.
- 🤔 The solution process involves choosing the correct 'x' value that makes sense in the context of the problem, avoiding negative concentrations.
- 📊 The final answer is the equilibrium molarity of O2, which is found to be 0.3 after rounding to one decimal place.
Q & A
What is the main topic of the video script?
-The main topic of the video script is calculating equilibrium composition from an equilibrium constant in a chemistry context.
Why is solving for X in this problem more challenging than usual?
-Solving for X in this problem is more challenging because the equilibrium constant (Kc) value is neither very large nor very small, which means the equilibrium does not favor one side or the other, and X cannot be treated as negligible.
What is the reaction taking place in the 500 milliliter flask as described in the script?
-The reaction taking place in the 500 milliliter flask is N2(g) + O2(g) ⇌ 2NO(g), which is the formation of nitrogen monoxide from nitrogen and oxygen gases.
What is the equilibrium constant expression for the given reaction?
-The equilibrium constant expression for the given reaction is Kc = [NO]^2 / ([N2] * [O2]), where the concentrations of N2 and O2 are in molarity.
What is the initial molarity of N2 and O2 in the flask, and how is it determined?
-The initial molarity of N2 is 0.200 M, and for O2, it is 1.000 M. These are determined by converting the given moles of N2 and O2 to molarity using the volume of the flask (500 mL or 0.500 L).
Why does the reaction shift to the left according to the script?
-The reaction shifts to the left because there is no O2 initially present in the flask, making it impossible to shift the reaction to the right to reach equilibrium.
What is the significance of the equilibrium constant value of 0.614 in this context?
-The equilibrium constant value of 0.614 indicates that the reaction is not strongly favoring the formation of products or the reactants, which is why the value of X cannot be neglected in the calculations.
What method is suggested in the script for solving the equilibrium problem when a calculator or software is not available?
-The script suggests using algebra and the quadratic formula as a method for solving the equilibrium problem when a calculator or software is not available.
What is the final equilibrium molarity of O2 that the script aims to find?
-The final equilibrium molarity of O2 that the script aims to find is 0.3 M, after solving for X using the quadratic formula and selecting the valid solution that does not result in negative concentrations.
Why is it incorrect to use the x value of 0.883 in the equilibrium calculations?
-Using the x value of 0.883 is incorrect because it results in a negative concentration for NO, which is not possible in real-world scenarios as concentrations cannot be negative.
What is the purpose of using an ICE table in this context?
-The purpose of using an ICE table in this context is to systematically keep track of the changes in concentrations of reactants and products as the reaction proceeds towards equilibrium, making it easier to set up and solve the equilibrium constant expression.
Outlines
🧪 Calculating Equilibrium Composition
The video script introduces a chemistry problem involving the calculation of equilibrium composition from an equilibrium constant. It emphasizes the complexity of solving for the variable 'X', which is not negligible in this scenario. The problem involves a reaction in a 500 milliliter flask with nitrogen (N2) and oxygen (O2) gases reacting to form nitrogen monoxide (NO). The equilibrium constant (KC) is given, and the script explains the importance of using molarity in the equilibrium expression. The initial step is to set up an ICE table (Initial, Change, Equilibrium) to understand how the reaction will shift towards equilibrium, considering the initial molarities of the reactants.
🔍 Solving the Quadratic Equation for Equilibrium
This section of the script delves into the process of solving the equilibrium problem without taking shortcuts, as the equilibrium constant (KC) is neither very large nor very small, indicating a balanced reaction. The script suggests using a calculator or an online solver for complex problems but also outlines the algebraic method involving the quadratic formula. It explains the steps to set up the quadratic equation based on the equilibrium expression and how to solve for 'X'. The script also discusses the importance of selecting the correct 'X' value that results in a physically meaningful concentration of reactants and products, ultimately arriving at the equilibrium molarity of O2.
📚 Detailed Algebraic Solution Using Quadratic Formula
The final paragraph of the script provides a detailed walkthrough of solving the equilibrium problem using algebra and the quadratic formula. It starts by rewriting the equilibrium constant expression and expanding it to form a standard quadratic equation. The script carefully guides through the algebraic manipulations, emphasizing the importance of accuracy to avoid mistakes. After forming the quadratic equation, the script uses the values of a, b, and c to apply the quadratic formula, resulting in two potential 'X' values. It concludes by selecting the valid 'X' value that corresponds to a positive and meaningful concentration of O2 at equilibrium, rounding the final answer to one decimal place as per the problem's requirement.
Mindmap
Keywords
💡Equilibrium Constant (Kc)
💡500 Milliliter Flask
💡Gaseous Reaction
💡ICE Table
💡Molarity
💡Quadratic Formula
💡Reaction Quotient
💡Significant Figures
💡Concentration
💡Equilibrium Molarity of O2
Highlights
Introduction to calculating equilibrium composition from an equilibrium constant.
Challenge of solving for X when it cannot be treated as negligible.
Description of a 500 milliliter flask containing N2 and O2 gases.
Reaction of N2 and O2 to form nitrogen monoxide (NO).
Writing out the equilibrium constant expression for KC.
Discussion on the significance of the value of KC in determining the position of equilibrium.
Use of an ICE table to determine initial concentrations and changes.
Initial molarity calculations for N2 and O2.
Direction of the reaction shift based on initial concentrations.
Equilibrium concentrations of N2, O2, and NO calculated using an ICE table.
Plugging equilibrium expressions into the equilibrium constant formula.
Explanation of why shortcuts are not possible in this problem.
Use of a quadratic formula to solve for X.
Discussion on the use of calculators or online solvers for solving equations.
Result of solving for X using algebraic methods.
Determination of the valid X value by checking against negative concentrations.
Final answer for the equilibrium molarity of O2.
Explanation of why molarities are used in ICE tables for direct answers.
Demonstration of solving the quadratic equation using algebra.
Final validation of the X value and its practical significance in the problem.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: