Chemistry_Calculating the Equilibrium constant
TLDRThe video script is a detailed lesson on chemical equilibrium, focusing on the calculation of the equilibrium constant (Kc). The instructor begins by introducing the concept of equilibrium in a closed system with reversible reactions, where the rates of the forward and reverse reactions are equal. The lesson then delves into the calculation of Kc using stoichiometric ratios and molar concentrations. The instructor uses a step-by-step approach to work through multiple problems, starting with initial conditions and changes in moles to find the equilibrium concentrations of reactants and products. Emphasis is placed on the inclusion of only gases or aqueous phase substances in Kc expressions, as solids and pure liquids are not included in such calculations. The lesson is practical, applying the concepts to hypothetical and past exam questions, illustrating the process with examples involving hydrogen, iodine, and nitrogen, as well as carbon dioxide and carbon monoxide. The summary underscores the importance of understanding the principles of chemical equilibrium and the meticulous process of calculating equilibrium constants, which is crucial for students of chemistry.
Takeaways
- 📚 The principle of chemical equilibrium is when the rate of the forward reaction equals the rate of the reverse reaction in a closed system, applicable to reversible reactions.
- ⚖️ Equilibrium constant (Kc) calculations involve the use of initial moles, changes in moles, and moles at equilibrium to determine the concentrations of reactants and products.
- 🧪 In calculations, the stoichiometric ratios from the balanced chemical equation are used to find the changes in moles of reactants and products at equilibrium.
- 📉 The concentration of a substance is calculated by dividing the number of moles by the volume of the container, which should be in cubic decimeters.
- 🚫 Solids and liquids are not included in Kc expressions because their concentration is constant and not variable like gases or aqueous solutions.
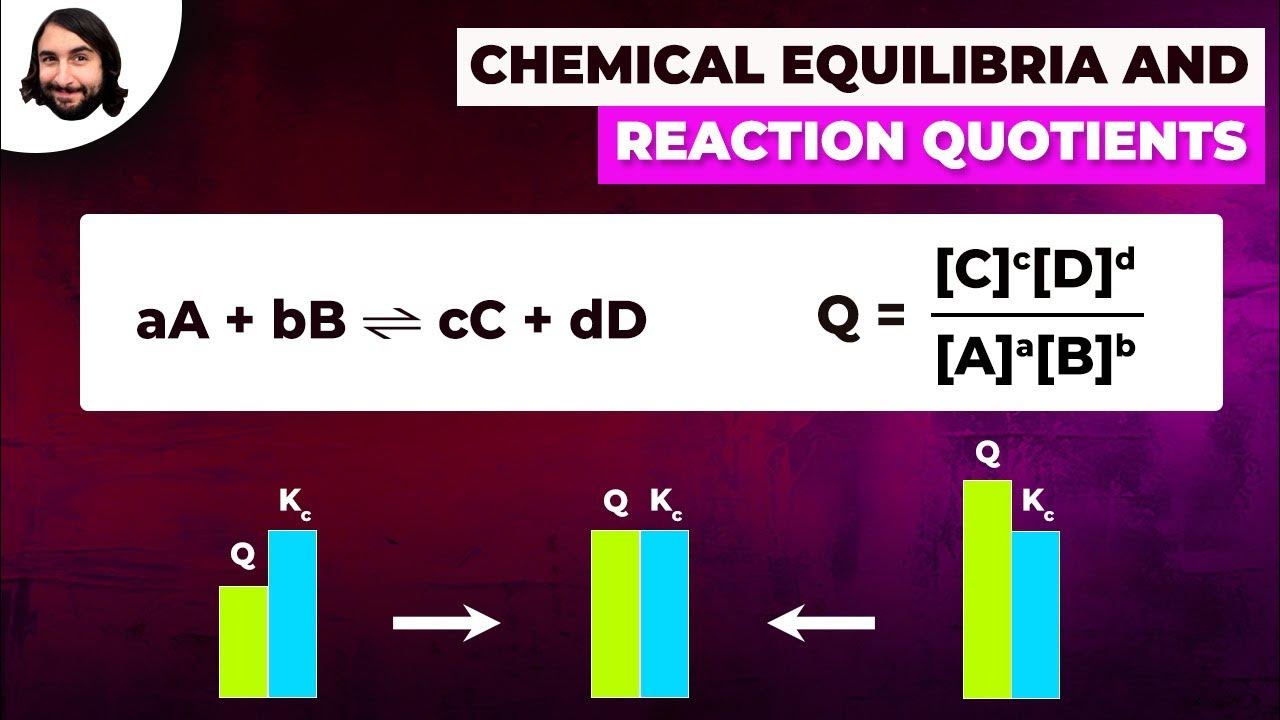
- 🔢 The Kc expression is written with the concentrations of products raised to the power of their stoichiometric coefficients divided by the concentrations of reactants also raised to their stoichiometric coefficients.
- 🧠 For Kc calculations, it is crucial to remember to substitute the equilibrium concentrations into the Kc expression without the units (moles per liter or cubic decimeters).
- 🔁 When the amount of a product is given at equilibrium, you can calculate the amount of reactants used and the remaining amount at equilibrium using stoichiometric ratios.
- ❗️ In equilibrium problems, if no initial amount for a product is given, it is assumed to be zero. Conversely, if no final amount for a reactant is given, it is assumed to be completely consumed.
- 📐 When converting units, ensure that all values are appropriately adjusted. For instance, cubic centimeters are converted to cubic decimeters by dividing by a thousand.
- 📝 Practice applying these principles to real past exam questions to solidify understanding and prepare for various problem types that might be encountered.
Q & A
What is the principle of calculating the equilibrium constant?
-The principle of calculating the equilibrium constant involves understanding the stoichiometry of the reaction, the initial amounts of reactants, and the changes that occur at equilibrium. The equilibrium constant (Kc) is calculated using the concentrations of products raised to the power of their stoichiometric coefficients divided by the concentrations of reactants raised to the power of their stoichiometric coefficients.
What are the conditions for a system to be at equilibrium?
-A system is at equilibrium when the rate of the forward reaction is equal to the rate of the reverse reaction. This occurs in a closed system where no matter or energy is entering or leaving, and it must involve a reversible reaction.
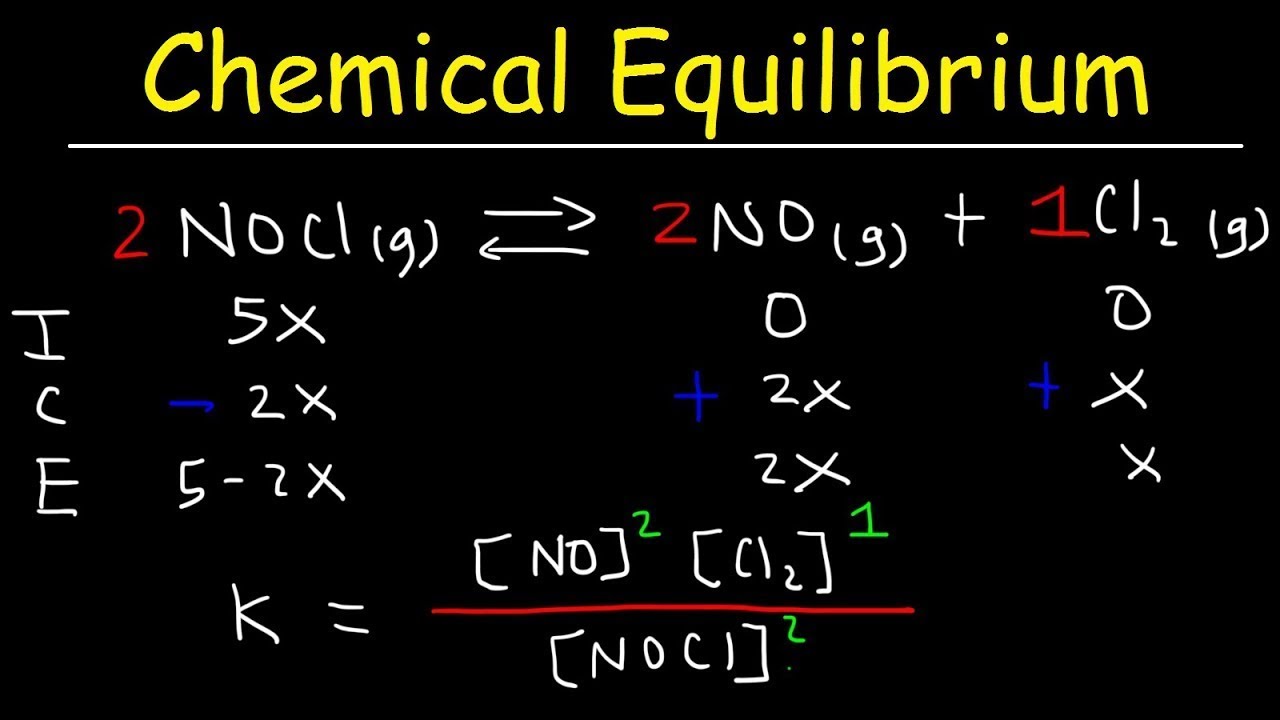
What does the ICE table represent in the context of equilibrium calculations?
-The ICE table stands for Initial, Change, and Equilibrium. It is a method used to keep track of the moles of reactants and products at different stages of a reaction, specifically the initial amounts, the change that occurs as the reaction proceeds, and the amounts at equilibrium.
How are the moles of a reactant at equilibrium calculated if the moles of a product are known?
-The moles of a reactant at equilibrium can be calculated using stoichiometric ratios from the balanced chemical equation. For every mole of product formed, a certain number of moles of reactants are consumed according to the reaction's coefficients. This ratio is used to determine the moles of reactants that have been used to produce the known amount of product.
Why are solids and pure liquids not included in the equilibrium expression?
-Solids and pure liquids are not included in the equilibrium expression because their concentration is constant and does not change during the reaction. The equilibrium constant expression only includes the concentration of reactants and products that are in the gaseous or aqueous phase.
What is the significance of the stoichiometric coefficients in writing the equilibrium expression?
-The stoichiometric coefficients from the balanced chemical equation indicate the ratio of moles of reactants and products involved in the reaction. In the equilibrium expression, the concentrations of the products are raised to the power of their stoichiometric coefficients and the concentrations of the reactants are raised to the power of their stoichiometric coefficients.
How do you calculate the concentration of a reactant or product when only the mass is given?
-To calculate the concentration of a reactant or product when only the mass is given, you first need to calculate the number of moles by dividing the mass by the molar mass of the substance. Then, you calculate the concentration by dividing the number of moles by the volume of the reaction container.
What is the Haber process mentioned in the script?
-The Haber process is an industrial method for the synthesis of ammonia from nitrogen and hydrogen gases. It is a key component in the production of fertilizers and is mentioned in the context of a chemical equilibrium problem involving nitrogen and hydrogen reacting to form ammonia.
How does the volume of the reaction container affect the equilibrium concentrations?
-The volume of the reaction container directly affects the equilibrium concentrations. According to the formula for concentration (c = n/V, where c is concentration, n is the number of moles, and V is volume), a larger volume results in lower concentrations and vice versa. This can shift the position of the equilibrium according to Le Chatelier's principle.
What is the equilibrium constant (Kc) for a reaction, and how is it used?
-The equilibrium constant (Kc) for a reaction is a measure of the extent to which a reversible reaction proceeds to completion. It is the ratio of the concentrations of products to the concentrations of reactants, each raised to the power of their stoichiometric coefficients. Kc is used to predict the direction in which a reaction will proceed and to calculate the concentrations of reactants and products at equilibrium.
How can you determine the amount of a reactant used in a reaction if the equilibrium constant and the concentrations at equilibrium are known?
-If the equilibrium constant (Kc) and the concentrations at equilibrium are known, you can use these values to work backwards to determine the amount of a reactant used. This involves setting up an expression using Kc and the equilibrium concentrations, solving for the unknown concentration of the reactant, and then calculating the change in moles from the initial amount to the equilibrium amount.
Outlines
📚 Introduction to Chemical Equilibrium Calculations
The speaker begins by addressing the audience and mentioning their first physics paper. They express anticipation for an upcoming lecture on chemistry, specifically focusing on chemical equilibrium calculations. The speaker intends to teach the principle of calculating the equilibrium constant and then analyze past exam questions for a deeper understanding.
🔍 Setting Up the Chemical Equilibrium Calculation
The speaker outlines a self-made question to demonstrate the calculation of the equilibrium constant. They start by establishing the initial moles of hydrogen and iodine, explaining the concept of chemical equilibrium in a closed system with reversible reactions. The scenario involves a reaction in a sealed container, and the goal is to calculate the equilibrium constant using the given moles of hydrogen iodide formed.
🧮 Calculating Changes in Moles for Equilibrium
The speaker continues the calculation process by addressing the changes in moles of reactants and products. They use stoichiometric ratios to determine the amounts of hydrogen and iodine that reacted to form hydrogen iodide. The calculation involves finding the moles of hydrogen used and the remaining moles of reactants at equilibrium, followed by calculating the equilibrium concentrations.
📉 Equilibrium Concentrations and Expression
The focus shifts to calculating the equilibrium concentrations of the reactants and products. The speaker emphasizes that only substances in the gaseous or aqueous phase are included in the equilibrium constant calculation. They derive the equilibrium expression for KC, highlighting the importance of using the correct stoichiometric coefficients.
🔢 Solving for the Equilibrium Constant (Kc)
The speaker provides a step-by-step solution for finding the equilibrium constant (Kc). They use the molar concentrations of the products and reactants at equilibrium, substituting the values into the Kc expression. The resulting Kc value is found to be 8.57, which is noted to be unitless.
🤔 Dealing with Unknowns in Equilibrium Calculations
The speaker tackles a question where the amount of one reactant is unknown. They guide through setting up the calculation table, considering the initial and equilibrium states, and using the given equilibrium constant to find the original amount of hydrogen. The process involves using stoichiometric ratios and the equilibrium constant to solve for the unknown.
🧪 Haber Process Example with Unknown Hydrogen
An example of the Haber process is given, where the reaction between nitrogen and hydrogen to form ammonia is discussed. The speaker uses the initial and equilibrium amounts of nitrogen to find the used and remaining amounts of hydrogen and ammonia. They calculate the equilibrium concentrations and use the equilibrium constant to find the original amount of hydrogen.
🧠 Advanced Calculation with Concentrations and Kc
The speaker presents a complex calculation involving the determination of the concentration of hydrogen and finding the value of an unknown amount of hydrogen (x). They use the equilibrium constant expression, substituting the concentration values and solving for x, which represents the initial moles of hydrogen.
📝 Calculation of Kc for a Given Reaction
The speaker concludes with a past exam question involving carbon dioxide and carbon monoxide. They calculate the initial moles of carbon dioxide from the given mass, determine the changes at equilibrium, and use the equilibrium constant to find the moles of carbon monoxide produced. The final step is calculating the Kc value using the molar concentrations of the products and reactants.
🏁 Conclusion and Invitation for Further Questions
The speaker wraps up the lesson by summarizing the process of calculating the equilibrium constant and addressing any remaining questions from the audience. They encourage students to reach out with further inquiries and thank them for their participation.
Mindmap
Keywords
💡Chemical Equilibrium
💡Equilibrium Constant (Kc)
💡Stoichiometric Ratios
💡Molar Concentration
💡Reversible Reaction
💡Initial Conditions
💡Gaseous Phase
💡Mole-to-Mole Relationships
💡Concentration Calculation
💡ICE Table
💡Haber Process
Highlights
Introduction to the topic of chemical equilibrium, focusing on equilibrium constant calculations.
Explanation of how to approach equilibrium constant calculations using moles and stoichiometric ratios.
Use of a self-made question to teach the principle of calculating chemical equilibrium.
Clarification on the importance of understanding the initial and equilibrium states in equilibrium problems.
Demonstration of how to set up and use an ICE (Initial, Change, Equilibrium) table for calculations.
Emphasis on the exclusion of solids and liquids from equilibrium constant calculations as their concentration cannot be determined.
Step-by-step calculation of the equilibrium constant for a given chemical reaction using moles and volume.
Illustration of how to convert cubic centimeters to cubic decimeters for volume calculations.
Solution to a problem involving an unknown amount of hydrogen reacting with nitrogen in the Haber process.
Use of the equilibrium constant value to calculate the original amount of hydrogen in a reaction.
Explanation of how to handle equilibrium calculations when the amount of a reactant is not given initially.
Application of stoichiometric ratios to find the change in moles of reactants and products.
Calculation of the equilibrium constant for a reaction involving carbon dioxide, carbon monoxide, and solid carbon.
Conversion of grams of carbon dioxide to moles for use in equilibrium calculations.
Determination of the equilibrium constant (Kc) for the given reaction using the concentration of carbon dioxide at equilibrium.
Final Kc value calculation results in 12.21 for the provided reaction at temperature T.
Offer to answer additional questions privately and encouragement for students to reach out for further clarification.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: