Practice Problem: ICE Box Calculations
TLDRThis educational script guides viewers through calculating equilibrium concentrations for a chemical reaction involving PCl5, PCl3, and Cl2. Starting with an initial concentration of 1.00 M for PCl5 and none for the products, the script uses an ICE table and the equilibrium constant Kc = 0.0211 to find the equilibrium concentrations. Through algebraic manipulation, it derives a quadratic equation, solves for the variable x, and determines the equilibrium concentrations as 0.865 M for PCl5, and 0.135 M for both PCl3 and Cl2, emphasizing the importance of positive solutions in chemical equilibrium.
Takeaways
- 🧪 The video discusses the calculation of equilibrium concentrations for a chemical reaction involving PCl5, PCl3, and Cl2.
- 📚 The initial concentration of PCl5 is given as 1.00 M, with PCl3 and Cl2 starting at 0 M, indicating the reaction starts solely with PCl5.
- 🔍 The equilibrium constant (Kc) for the reaction is provided as 0.0211, which is crucial for determining the equilibrium concentrations.
- 📉 The ICE (Initial, Change, Equilibrium) table is introduced as a method to set up and solve for equilibrium concentrations.
- 🔄 The reaction proceeds in the forward direction as only the reactant (PCl5) is initially present, and products (PCl3 and Cl2) are formed.
- ⚖️ For every one mole of PCl5 that reacts, one mole each of PCl3 and Cl2 is produced, maintaining a 1:1:1 stoichiometric ratio.
- 📝 The equilibrium expression is set up with the concentrations of the products in the numerator and the concentration of the reactant in the denominator, all to the power of 1.
- 🔢 An algebraic approach is used to solve for the variable 'x', representing the change in concentration of each species at equilibrium.
- 🔧 The quadratic formula is applied to solve the resulting quadratic equation for 'x', yielding two potential solutions.
- 📉 Only the positive solution for 'x' is physically meaningful, as negative concentrations would be illogical in this context.
- 📊 The equilibrium concentrations are calculated as 0.865 M for PCl5 and 0.135 M for both PCl3 and Cl2, based on the positive value of 'x'.
- 📚 The video encourages viewers to refer to tutorials on chemical equilibria and algebra for a deeper understanding of the concepts.
Q & A
What is the initial concentration of PCl5 given in the script?
-The initial concentration of PCl5 is given as 1.00 molar.
What are the initial concentrations of PCl3 and Cl2 in the script?
-The initial concentrations of both PCl3 and Cl2 are 0, indicating that they are not present at the start of the reaction.
What is the equilibrium constant (Kc) for the given reaction?
-The equilibrium constant (Kc) for the reaction is 0.0211.
What is the stoichiometric ratio of the reactants and products in the reaction?
-The stoichiometric ratio of the reactants and products is 1:1:1, meaning for every one molecule of PCl5 that reacts, one molecule of PCl3 and one molecule of Cl2 are produced.
What is an ICE box and how is it used in the script?
-An ICE box is a table used to set up the initial, change, and equilibrium concentrations for a chemical reaction. In the script, it is used to calculate the equilibrium concentrations of PCl5, PCl3, and Cl2.
What algebraic method is suggested in the script to solve for the equilibrium concentrations?
-The script suggests using the quadratic formula to solve for the equilibrium concentrations.
How does the script handle the two possible solutions from the quadratic equation?
-The script explains that only the positive solution from the quadratic equation makes sense in the context of the chemical reaction, as a negative solution would imply concentrations greater than the initial amount or negative concentrations, which is not feasible.
What is the equilibrium concentration of PCl5 after the reaction, according to the script?
-The equilibrium concentration of PCl5 is calculated to be 0.865 molar after the reaction.
What are the equilibrium concentrations of PCl3 and Cl2 after the reaction, according to the script?
-The equilibrium concentrations of both PCl3 and Cl2 are calculated to be 0.135 molar after the reaction.
What is the significance of the equilibrium constant (Kc) in the script?
-The equilibrium constant (Kc) is significant as it is used to determine the extent of the reaction at equilibrium. It helps in setting up the equation to find the equilibrium concentrations of the reactants and products.
Why is it necessary to convert the equilibrium expression into a quadratic equation in the script?
-Converting the equilibrium expression into a quadratic equation is necessary to solve for the variable x, which represents the change in concentration of the reactants and products, allowing the calculation of the equilibrium concentrations.
Outlines
🧪 Calculating Equilibrium Concentrations
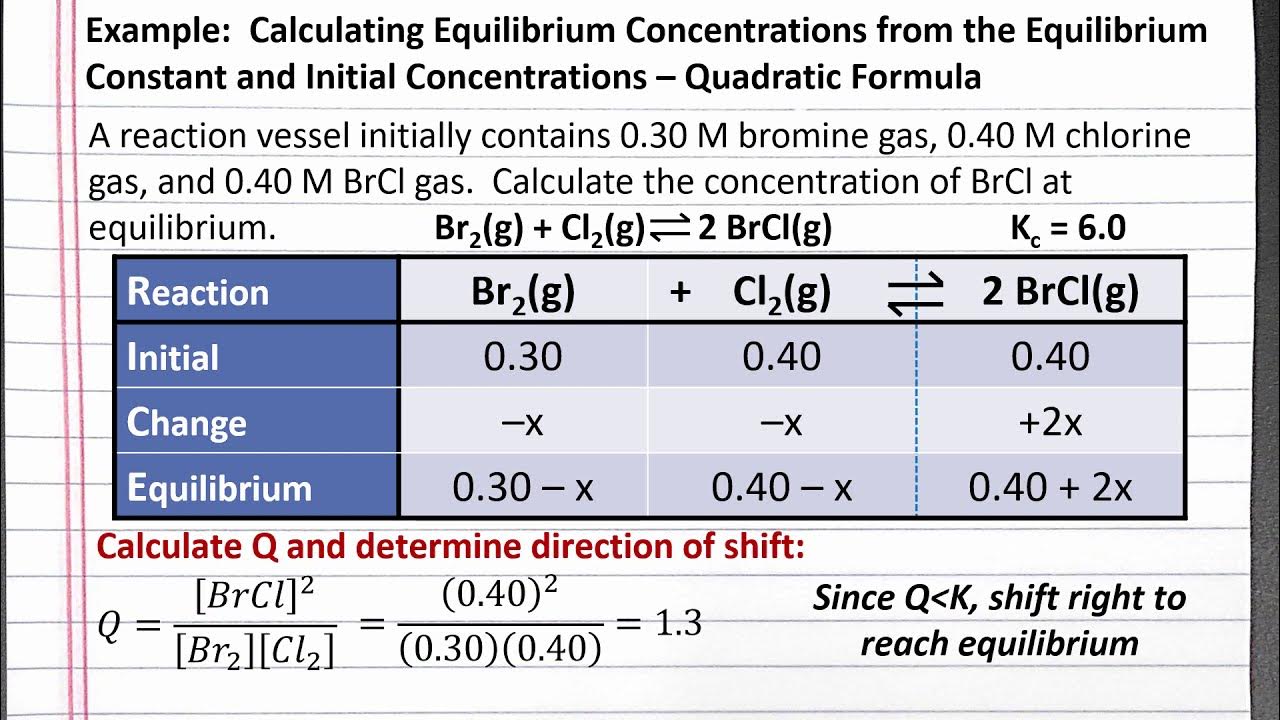
This paragraph introduces the process of calculating equilibrium concentrations for a chemical reaction involving PCl5, PCl3, and Cl2. It starts with an initial concentration of 1.00 M for PCl5 and 0 M for both PCl3 and Cl2, indicating that the reaction begins solely with PCl5. The equilibrium constant (Kc) for the reaction is given as 0.0211. The paragraph explains the setup of the ICE (Initial, Change, Equilibrium) table and the stoichiometric coefficients, which are all 1 in this case, leading to a straightforward calculation. The goal is to find the equilibrium concentrations of all three substances by solving an algebraic equation derived from the Kc expression.
🔍 Solving for Equilibrium Concentrations
The second paragraph continues the discussion on calculating equilibrium concentrations, focusing on solving the derived algebraic equation. The method involves multiplying both sides of the Kc equation by the concentration of PCl5 and rearranging terms to form a quadratic equation. The quadratic formula is mentioned as a tool for solving such equations, with the coefficients A, B, and C identified from the equation. The solutions for X are calculated, and the positive solution is chosen as it makes physical sense in the context of the chemical reaction. The equilibrium concentrations for PCl5, PCl3, and Cl2 are then determined to be 0.865 M, 0.135 M, and 0.135 M, respectively. The paragraph concludes by emphasizing the use of the ICE table in conjunction with the Kc expression to find the equilibrium concentrations.
Mindmap
Keywords
💡Equilibrium Concentrations
💡PCl5
💡PCl3
💡Cl2
💡Equilibrium Constant (Kc)
💡ICE Table
💡Stoichiometric Coefficients
💡Quadratic Equation
💡Quadratic Formula
💡Algebra
💡Chemical Equilibria
Highlights
Practice calculating equilibrium concentrations with the given chemical equilibrium system involving PCl5, PCl3, and Cl2.
Initial concentration of PCl5 is 1.00 M, with PCl3 and Cl2 starting at 0 M.
Equilibrium constant (Kc) for the system is given as 0.0211.
Introduction to the ICE (Initial, Change, Equilibrium) table method for determining equilibrium concentrations.
Stoichiometric coefficients are all 1, simplifying the ICE table setup.
The forward reaction is assumed due to the presence of reactant and absence of products initially.
For every 1 mole of PCl5 that reacts, 1 mole each of PCl3 and Cl2 is produced.
Equilibrium concentrations are calculated by adding initial and change values.
Algebraic manipulation is required to solve for the variable X in the equilibrium expression.
Quadratic equation is formed from the equilibrium constant expression.
Quadratic formula is introduced as a method to solve for X.
Two solutions are obtained from the quadratic equation, but only the positive value is physically meaningful.
The positive solution for X is 0.135 M, indicating the amount of PCl5 that has reacted.
Final equilibrium concentrations are calculated: PCl5 at 0.865 M, and PCl3 and Cl2 both at 0.135 M.
The ICE table method is effectively demonstrated for calculating equilibrium concentrations in a chemical system.
The tutorial provides a step-by-step guide for students to understand and apply chemical equilibrium calculations.
The importance of selecting the correct solution from the quadratic equation in the context of chemical equilibrium is emphasized.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: