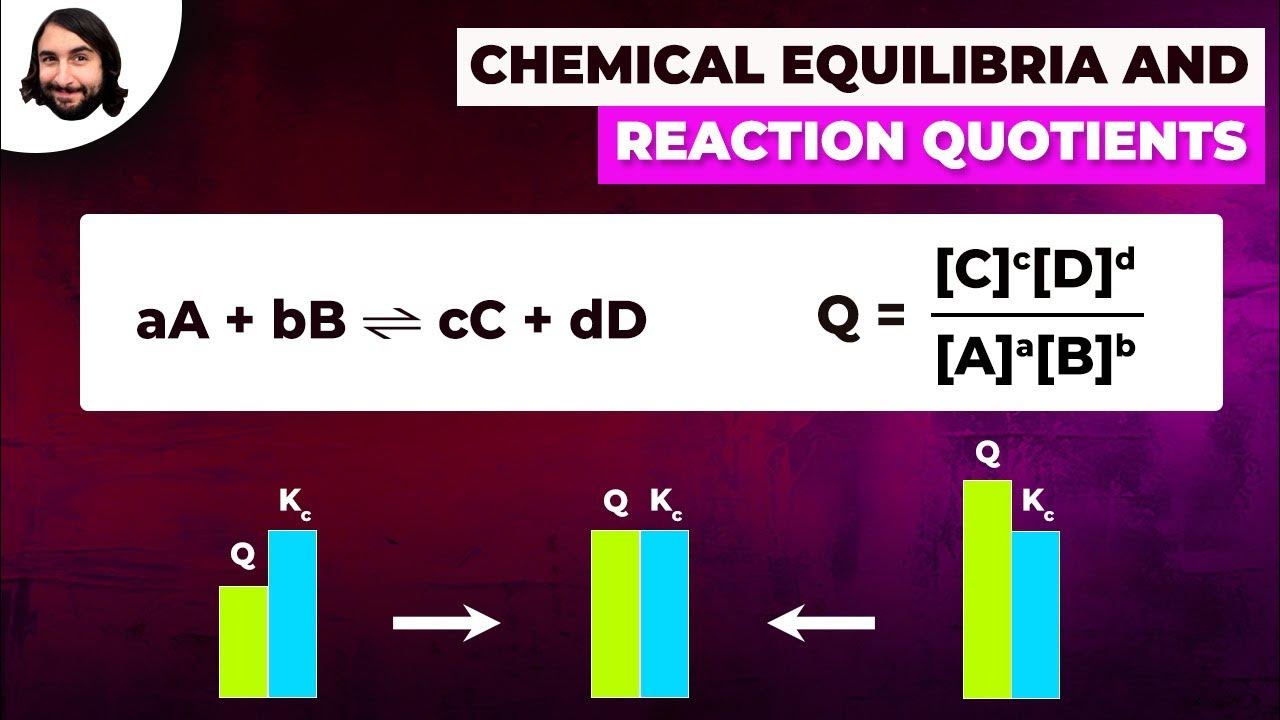
Using RICE to calculate equilibrium concentrations
TLDRThe video script discusses the calculation of the equilibrium constant (Kc) for a reversible reaction when equilibrium concentrations are not given. It introduces the RICE method, which stands for Ratio of Initial to Equilibrium moles, Change in moles, and Equilibrium moles. The example problem involves the reaction between nitrogen monoxide (NO) and oxygen (O2) to form nitrogen dioxide (NO2). The script provides a step-by-step process to determine the equilibrium moles and concentrations of the reactants and products, and then uses these values to calculate Kc. The method involves setting up a RICE table, calculating changes in moles based on the balanced chemical equation, converting moles to concentrations using the volume of the container, and finally plugging the concentrations into the Kc expression. The example concludes with the calculation of Kc as 45 moles to the power of minus one decimeters cubed.
Takeaways
- 🧪 To calculate the equilibrium constant (Kc) for a reversible reaction without equilibrium concentrations, the Rice method is used.
- 🔍 The Rice method involves creating a table with columns for Ratio of moles (R), Initial moles (I), Change in moles (C), and Equilibrium moles (E).
- ⚖️ The balanced chemical equation is used to determine the ratio of moles for the reactants and products.
- 📝 Initial moles are the amounts of reactants before the reaction starts.
- ⚖️ At equilibrium, the moles of products formed and reactants consumed are given.
- 🔄 The change in moles is calculated by subtracting initial moles from equilibrium moles for reactants and vice versa for products.
- 🧮 Equilibrium moles are found by applying the change in moles to the initial moles.
- 🏗️ The volume of the container is used to convert moles to molar concentrations (moles per decimeter cubed) by dividing the number of moles by the volume.
- 📐 The Kc expression is derived from the balanced equation, with concentrations of products in the numerator and reactants in the denominator, each raised to the power of their stoichiometric coefficients.
- 🧮 Plug the equilibrium concentrations into the Kc expression to solve for Kc.
- 📊 The units for Kc are derived from the concentrations used, simplifying to moles to the power of -1 times decimeters cubed (mol^-1·dm^3).
- 🎓 Example calculation results in a Kc value of 45 mol^-1·dm^3 for the given scenario.
Q & A
What is the balanced chemical equation for the reaction between nitrogen monoxide and oxygen?
-The balanced chemical equation for the reaction is 2NO + O2 ⇌ 2NO2, indicating that two moles of nitrogen monoxide react with one mole of oxygen to produce two moles of nitrogen dioxide.
What is the Rice method used for in the context of this script?
-The Rice method is used to calculate the equilibrium concentrations when the equilibrium concentrations are not directly given. It involves determining the initial moles, the change in moles, and the equilibrium moles for each species involved in the reaction.
What are the units for the equilibrium constant (Kc) in the context of this problem?
-The units for the equilibrium constant (Kc) in this context are moles to the power of -1 decimeters cubed (mol^-1 dm^3), as the concentrations are expressed in moles per decimeter cubed.
How do you calculate the change in moles for the reactants and products in the Rice method?
-You calculate the change in moles by comparing the initial moles and the equilibrium moles. For products, the change is the difference between the equilibrium moles and the initial moles (which is zero for products at the start). For reactants, it is the difference between the initial moles and the equilibrium moles.
What is the ratio of moles in the Rice method and how is it determined?
-The ratio of moles in the Rice method refers to the stoichiometric coefficients from the balanced chemical equation. It is determined by looking at the balanced numbers of moles of each species that react or are produced.
How do you find the equilibrium moles of the reactants and products in the Rice method?
-You find the equilibrium moles by starting with the initial moles, then applying the changes in moles (which are calculated based on the ratio of moles and the known change for one of the products or reactants) to determine the final amount of each species at equilibrium.
What is the initial moles of nitrogen monoxide and oxygen given in the problem?
-The initial moles of nitrogen monoxide (NO) is 1.6 moles, and the initial moles of oxygen (O2) is 1.4 moles.
At equilibrium, how many moles of nitrogen dioxide are formed?
-At equilibrium, 1.2 moles of nitrogen dioxide (NO2) are formed.
What is the equilibrium concentration of NO2, NO, and O2 in moles per decimeter cubed?
-The equilibrium concentrations are 0.3 moles per decimeter cubed for NO2, 0.1 moles per decimeter cubed for NO, and 0.2 moles per decimeter cubed for O2.
How do you convert moles to moles per decimeter cubed?
-To convert moles to moles per decimeter cubed, you divide the number of moles by the volume of the container in decimeters cubed.
What is the calculated value of Kc for the given reaction at equilibrium?
-The calculated value of Kc for the given reaction at equilibrium is 45 moles to the minus one decimeters cubed (45 mol^-1 dm^3).
What does the 'RICE' table consist of in the context of this script?
-The 'RICE' table consists of four parts: 'R' stands for the ratio of moles (from the balanced equation), 'I' stands for initial moles, 'C' stands for the change in moles (calculated based on the reaction progress), and 'E' stands for equilibrium moles.
Outlines
🧪 Introduction to the Rice Method for Equilibrium Calculations
The paragraph introduces the Rice method, a technique used to calculate equilibrium concentrations when not given directly. It sets up a scenario involving the reversible reaction of nitrogen monoxide and oxygen to form nitrogen dioxide. The initial moles of reactants and the volume of the container are provided, along with the equilibrium moles of the product. The goal is to determine the equilibrium moles and concentrations of all species to subsequently calculate the equilibrium constant (Kc). The explanation outlines the steps to set up the Rice table, which includes the ratio of moles, initial moles, equilibrium moles, and the change in moles.
🔍 Applying the Rice Method to Find Equilibrium Moles
This paragraph delves into applying the Rice method to the given chemical scenario. It explains how to use the balanced chemical equation to find the ratio of moles, which is then used to determine the initial and equilibrium moles of reactants and products. The paragraph walks through the calculation of changes in moles for reactants and products based on the stoichiometry of the reaction. It concludes with the computation of equilibrium moles for nitrogen monoxide and oxygen, and the conversion of these moles to equilibrium concentrations by dividing by the volume of the container.
🧠 Calculating Kc with Equilibrium Concentrations
The final paragraph focuses on the calculation of the equilibrium constant (Kc) using the equilibrium concentrations obtained from the previous step. It details the formulation of the Kc expression according to the balanced equation, emphasizing that concentrations (moles per decimeter cubed) are used rather than moles. The paragraph concludes with the insertion of the calculated concentrations into the Kc expression, resulting in a Kc value of 45 with units of moles to the minus one decimeters cubed, after simplifying the expression.
Mindmap
Keywords
💡Reversible reaction
💡Equilibrium concentrations
💡Rice method
💡Balanced equation
💡Initial moles
💡Equilibrium moles
💡Change in moles
💡Equilibrium constant (Kc)
💡Mole ratio
💡Concentration
💡Volume of the container
Highlights
The transcript discusses calculating the equilibrium constant (Kc) for a reversible reaction using equilibrium concentrations.
If equilibrium concentrations are not given, the Rice method is introduced to calculate them.
A balanced chemical equation involving nitrogen monoxide and oxygen is provided as an example.
Initial moles of reactants and the volume of the container are given, along with the equilibrium moles of the product.
The RICE method is explained, which stands for Ratio, Initial, Change, and Equilibrium moles.
The ratio of moles is derived from the balanced equation's stoichiometric coefficients.
Initial moles of NO and O2 are given as 1.6 and 1.4 respectively.
At equilibrium, 1.2 moles of NO2 are formed, indicating the change in moles for the product.
The change in moles for reactants is calculated using the ratio from the balanced equation.
Equilibrium moles for all species are determined by applying the changes to the initial moles.
Concentrations are found by converting moles to moles per decimeter cubed using the container's volume.
The Kc expression is derived from the balanced equation, including all gases as they are in the same phase.
Equilibrium concentrations are plugged into the Kc expression to calculate its value.
The calculated Kc value for the given reaction is 45 with units of moles to the minus one decimeters cubed.
The importance of understanding changes in moles and their relation to reactants and products in a reversible reaction is emphasized.
The process of converting moles to concentrations using the volume of the container is detailed.
The transcript provides a step-by-step guide on how to apply the Rice method to find Kc without given equilibrium concentrations.
The final Kc expression and its units are derived from the equilibrium concentrations of the reactants and products.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: